
Joint replacement surgeries offer a new lease on life for countless individuals grappling with debilitating pain and limited mobility due to conditions like osteoarthritis, osteonecrosis, and rheumatoid arthritis. These medical advancements promise years of improved quality of life, allowing patients to regain their independence and participate in daily activities without constant discomfort. However, the trust placed in medical device manufacturers is paramount, as the integrity of these implanted devices directly impacts patient well-being and long-term health outcomes. When that trust is eroded by defective products, the consequences can be devastating, leading to unforeseen complications, repeated surgeries, and profound distress for those who sought relief.
In recent years, the medical community and hundreds of thousands of patients worldwide have been confronted with a significant alert regarding Exactech joint replacement devices. This Florida-based manufacturer, once a growing global entity specializing in surgical implants for joint replacements, has become the focal point of a widespread recall and a deluge of lawsuits. The core issue revolves around defective packaging that has compromised the integrity of knee, ankle, and hip implants, leading to premature wear, failure, and a host of painful symptoms for patients. The situation has underscored the critical importance of rigorous manufacturing standards, transparent reporting, and robust consumer protections in the medical device industry.
As this complex saga unfolds, it is crucial for consumers, healthcare providers, and anyone with an Exactech implant to understand the underlying issues, the scope of the problem, and the available avenues for recourse. This in-depth guide aims to provide objective, evidence-based information, empowering you with the knowledge needed to navigate this challenging medical alert. We will delve into the company’s financial struggles, the specific nature of the packaging defect, the widespread recalls, and the official recommendations from regulatory bodies like the FDA, offering a clear perspective on a situation that has impacted so many lives.

1. **Exactech’s Journey to Bankruptcy**Exactech, once a thriving device manufacturer established by orthopedic surgeons in the 1980s, grew over three decades from a small operation into a significant global entity. The company specialized in surgical implants for joint replacements, including popular lines like Optetrak, Logic, and Truliant for knees, Vantage for ankles, and Novation, Acumatch, and MCS for hips. However, this trajectory of growth took a sharp turn as the company faced an escalating number of lawsuits from patients alleging defective implants.
By October 2024, the mounting litigation costs, associated with thousands of claims of defective knee, hip, and ankle implants, became unsustainable. Exactech ultimately filed for Chapter 11 bankruptcy protection in federal court in Delaware. This strategic move, announced by Exactech’s president and chief executive officer Darin Johnson, aimed to restructure the company and secure financing, with an investor group providing approximately $85 million to fund ongoing operations.
The bankruptcy filing, however, was met with dismay by attorneys representing injured patients. Joe Saunders, a Florida attorney, characterized it as “a slap in the face to all the joint-implant patients and doctors who trusted the company,” emphasizing the special responsibility a medical device company holds for public health. He further suggested that the bankruptcy “serves to cover up public disclosure of the company putting profits ahead of safety,” especially as it paused public trials that were set to begin.
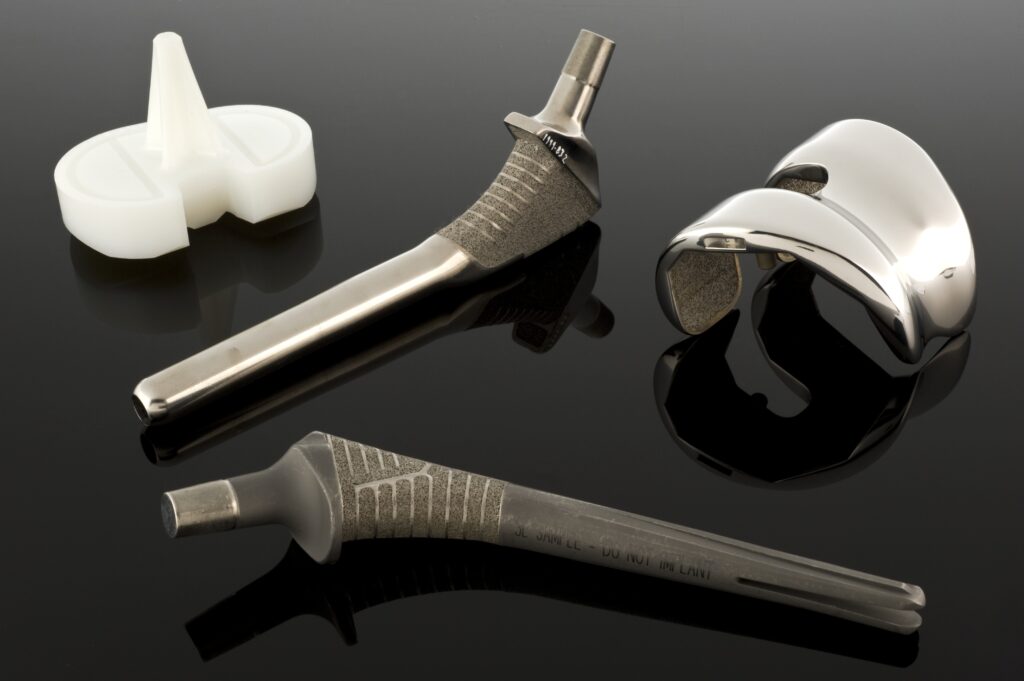
2. **The Core Defect: Faulty Packaging Leading to Oxidation**At the heart of the Exactech recall crisis lies a critical manufacturing flaw in the packaging of many of its joint replacement devices. Exactech joint replacement devices, used to treat painful, arthritic joints, all contain a plastic component designed to be protected within packaging featuring multiple oxygen barrier layers. This sophisticated packaging is essential to prevent a chemical reaction known as oxidation, which can severely degrade the plastic (polyethylene) component over time.
Exactech’s investigation revealed that the defective packaging bags were missing one of these vital oxygen barrier layers. This omission allowed oxygen from the air to contact the plastic component prematurely, before the device was even implanted into a patient’s body. The pre-implantation oxidation significantly compromised the plastic’s integrity, leading to a host of problems once the device was in use.
The consequences of this oxidation are severe and far-reaching. It can lead to accelerated device wear, component cracking or fracture, and ultimately, device failure. For patients, this translates to new or worsening pain, more bone loss, swelling in the affected area, and often, the necessity of corrective revision surgery to remove and replace the failed implant. The defect undermines the very purpose of the implant – to provide long-lasting relief and mobility – by causing it to degrade far sooner than its typical 15 to 20-year lifespan.
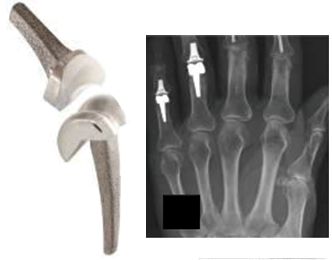
3. **The Scope of the Recalls: Impacting Knees, Ankles, and Hips**Exactech initiated a series of voluntary recalls for its artificial knees, hips, and ankles, beginning in August 2021. The initial blame was placed on a packaging defect dating back as far as 2004, which was found to cause the plastic component to wear out prematurely in approximately 140,000 implants. This broad scope highlights a systemic issue that affected a substantial portion of the company’s product lines over an extended period.
For knee and ankle replacement devices, Exactech expanded a voluntary recall on February 7, 2022, encompassing all Optetrak, Logic, and Truliant knee replacements, as well as Vantage total ankle replacements. This recall applied regardless of a device’s label or shelf life, because around 80% of these knee and ankle replacement devices manufactured since 2004 were packaged in defective bags. The devices included specific components like various CR and PS Tibial Inserts for Optetrak, Optetrak Logic, and Truliant knees, and the Fixed-Bearing Liner Component for Vantage ankles.
Hip replacement devices were also significantly impacted. In June 2021, Exactech first recalled some GXL Liners for Novation, Acumatch, and MCS hip replacement devices due to premature and excessive wear. Initially, the root cause was unknown. However, by August 2022, Exactech expanded this hip replacement device recall to include all hip devices with polyethylene components packaged in the defective bags, solidifying the packaging defect as the overarching problem. More recently, by March 2024, Exactech voluntarily recalled its Equinoxe Shoulder System implants for the same packaging defects, indicating a wider-reaching problem across multiple product categories.

4. **FDA’s Critical Safety Alert and Patient/Provider Recommendations**The U.S. Food and Drug Administration (FDA) has played a crucial role in disseminating information and guiding both patients and healthcare providers through the Exactech recall. On March 23, 2023, the FDA issued a safety communication, reminding the public about Exactech joint replacement devices manufactured between 2004 and August 2021 that were recalled in 2021 and 2022. This communication reinforced the critical issue of defective packaging, which allowed oxygen to degrade the plastic components and increase the risk of accelerated device wear and failure.
For patients, the FDA offers clear, actionable recommendations. If an Exactech knee, ankle, or hip replacement device is functioning well and without pain or symptoms, the FDA does not recommend surgery for its removal. However, it strongly advises patients with any Exactech joint replacement device to contact their healthcare provider immediately if they experience any new or worsening pain, swelling, inability to bear weight, grinding or other noises, or weakness around the implanted device. The FDA also highlighted Exactech’s online database for knee and ankle devices, which patients can use with their serial number to check if their implant is part of the recall.
Healthcare providers also received specific instructions. The FDA clearly stated that no recalled Exactech knee, ankle, or hip devices should be implanted. Similar to patient advice, the FDA does not recommend routine removal of well-functioning Exactech devices in asymptomatic patients. However, they are urged to monitor patients with implants manufactured between 2004 and August 2021 for signs of wear, failure, or bone loss. This includes considering X-rays if a failed device is suspected. Furthermore, revision surgery should be discussed on a case-by-case basis with patients experiencing worsening symptoms, with a focus on shared decision-making regarding benefits and risks. All recalled devices in inventory must be removed and returned to Exactech. The FDA continues to work with Exactech and international regulatory agencies to assess risks and ensure patient safety.

5. **Patient Impact: The Painful Reality of Injuries and Revision Surgeries**The defective Exactech implants have caused a range of severe and painful complications for thousands of patients who had hoped for restored mobility and relief from chronic pain. When these devices fail prematurely due to component degradation, the impact on a patient’s physical health and quality of life can be profound. Many recipients have faced symptoms that include new or worsening pain, significant bone loss, and debilitating problems with mobility.
These issues can manifest as difficulty walking, an inability to bear weight on the affected joint, and instability. Patients often report alarming noises such as clicking, grinding, or popping coming from the implant area. Such symptoms are clear indicators of device failure, component fracture, or excessive wear, all stemming from the oxidation caused by the faulty packaging. The experience is often described as a return to the very conditions the original surgery was meant to alleviate, sometimes even worse.
For many, the only solution to these debilitating problems is to undergo a second, often more complex and painful, corrective revision surgery. This means another hospitalization, another period of recovery, and the inherent risks associated with any surgical procedure, including infection and further complications. The physical toll is immense, as exemplified by cases like Michael Giordano, who, after receiving an Exactech knee implant in 2015, experienced severe problems by 2020. Even after a revision surgery with another recalled implant, he continues to suffer severe pain, swelling, difficulty walking, balance issues, and ongoing bone and soft tissue damage, necessitating yet another future surgery.
6. **Surgeon and Patient Reactions: Outrage and Disappointment Over Trust Betrayed**The widespread recalls and the subsequent revelations about Exactech’s defective implants have understandably ignited strong reactions from both the legal community representing injured patients and the patients themselves. The trust placed in medical device manufacturers is foundational, and the feeling of betrayal runs deep when that trust is violated by products designed to alleviate suffering, not create more.
Joe Saunders, a Florida attorney actively litigating against Exactech, voiced a common sentiment among legal representatives when he stated that the bankruptcy filing was “a slap in the face to all the joint-implant patients and doctors who trusted the company.” He underscored the moral imperative of medical device companies to uphold public health above all else, suggesting that Exactech had prioritized profits over safety. The abrupt halt of public trials due to bankruptcy proceedings further fueled concerns that the company was attempting to conceal the full truth of its conduct.
Patients, who live with the direct consequences of these implant failures, express profound anger and frustration. Sue Sacher, a 76-year-old New Jersey resident, articulated this rage, questioning, “How did they [Exactech] think they are not responsible for this?” After having both knees replaced with Exactech implants in 2006 and 2009, she endured the painful necessity of having both implants replaced again. Her experience, mirroring that of many others, highlights the significant physical and emotional burden placed upon individuals who suffer from these defective devices.

7. **Exactech’s Initial Response and Statements**In the face of escalating litigation and widespread recalls, Exactech has issued statements regarding its position and commitment. Darin Johnson, Exactech’s president and chief executive officer, acknowledged the “unsustainable liabilities associated with knee and hip litigation related to the packaging recalls we voluntarily initiated between 2021 and 2022.” This statement, released concurrently with the company’s bankruptcy filing, directly links the financial distress to the defective product lawsuits.
Despite the acknowledgment of liabilities, Exactech has maintained its operational status during the bankruptcy proceedings. Johnson also stated, “We take our commitment to patient well-being very seriously and have provided substantial out-of-pocket patient reimbursements and surgeon support for related expenses.” This assertion indicates the company’s efforts to address some of the immediate financial burdens faced by patients and medical professionals due to the recalls.
However, the company has consistently denied the allegations of an “unacceptable failure and complication rate” made in many of the lawsuits. Exactech had no comment on the lawsuits themselves, typically a legal strategy during such complex litigation. The disparity between Exactech’s claims of serious commitment to patient well-being and the lawyers’ accusations of prioritizing profits over safety underscores the contentious nature of the ongoing legal and ethical debate surrounding the company’s actions. The company’s communication has aimed to project responsibility while navigating immense financial and legal pressure.

8. **The Consolidated Lawsuits: MDL 3044 and the Path to Justice**Multidistrict litigation (MDL) represents a critical legal mechanism designed to streamline the handling of numerous similar lawsuits filed in different federal courts across the country. This centralized approach allows for more efficient management of complex product liability lawsuits, such as those brought against Exactech, by consolidating them into a single federal court. In October 2022, the Panel on Multidistrict Litigation approved the request to consolidate Exactech cases, transferring them to the Eastern District of New York. U.S. District Judge Nicholas G. Garaufis was assigned to oversee these coordinated proceedings, a strategic move intended to centralize discovery, pretrial motions, and potentially, settlements, thereby benefiting both the judicial system and the plaintiffs seeking redress.
As of October 2025, a substantial number of individuals, specifically 1,838 Exactech lawsuits, were pending in MDL 3044, reflecting the widespread impact of defective Exactech joint replacement devices. However, the legal landscape for these cases underwent a significant transformation in October 2024 when Exactech filed for Chapter 11 bankruptcy protection. This corporate action triggered an automatic stay, which temporarily paused most ongoing lawsuits, including those within the federal MDL and many state court cases, as the company entered a period of financial restructuring.
Prior to the bankruptcy filing, the MDL was actively progressing towards bellwether trials. These are test cases strategically selected to be tried first, offering all parties involved an indication of how juries might react to specific evidence and legal arguments. Initially, Gayle Tarloff’s trial was set to begin in June 2025, with Geraldine Larson’s trial scheduled for August 2025. Following subsequent discovery disputes, these dates were moderately delayed to July 6, 2025, and September 29, 2025, respectively. Nevertheless, Exactech’s bankruptcy filing further complicated these trial dates, placing them in an uncertain status; as of now, no trials or approved settlements in MDL 3044 have been recorded.
These bellwether trials play a pivotal role in MDLs, serving as a crucial mechanism for plaintiffs and defendants to evaluate potential jury reactions and to assess the strengths and weaknesses of their respective arguments. The outcomes from these initial trials often serve to inform and shape subsequent settlement negotiations for the broader pool of consolidated cases. The pause imposed by the bankruptcy has undoubtedly caused frustration for many plaintiffs who had been anticipating these trials as a vital step toward resolution and justice. Despite the automatic stay, already-filed claims continue to navigate the legal system, albeit at a modified and often slower pace, as the bankruptcy proceedings unfold.
9. **Allegations Against Exactech: Unpacking Claims of Negligence and Failure to Warn**Patients who have initiated lawsuits against Exactech are not merely alleging product failure; they contend that the company sold defective hip, knee, and ankle implants with prior knowledge of their flaws, pointing to a perceived lack of corporate responsibility and transparency. The core legal claims brought against Exactech are comprehensive, encompassing allegations of breach of warranty, failure to warn, fraud, negligence, and strict product liability. These claims collectively assert that Exactech either knowingly distributed dangerous products or fundamentally failed in its duty to ensure product safety and adequately inform both patients and medical professionals about the significant potential risks associated with its implants.
Among the central allegations are claims of breach of warranty, which suggest that Exactech’s products failed to meet implied or express promises regarding their quality, durability, and fitness for their intended use, thereby failing to deliver the expected longevity and performance. Concurrently, strict product liability claims hold manufacturers accountable for injuries caused by defective products, irrespective of fault, simply because the product itself was defective and caused harm. This legal principle is frequently invoked in medical device litigation, as it focuses on the inherent flaw of the product rather than requiring proof of the manufacturer’s specific intent or knowledge.
The lawsuits further detail claims of negligence, asserting that Exactech failed to exercise reasonable care throughout the design, manufacturing, or marketing phases of its implants, directly leading to patient harm. This alleged negligence could manifest in various forms, including insufficient testing protocols, inadequate quality control measures, or a delayed and insufficient response to known product issues. Perhaps even more critically, “failure to warn” claims contend that Exactech did not adequately inform medical providers and patients about the significant risks stemming from the defective packaging and the premature degradation of the implants. This alleged omission prevented surgeons from making fully informed decisions when implanting the devices and denied patients a complete understanding of the potential complications they might face.
Accusations of fraud suggest that Exactech may have intentionally misrepresented the safety or efficacy of its products or actively concealed known defects. The sheer scale of the problem is highlighted by Exactech’s own April 2022 Urgent Medical Device Correction letter, which indicated that at least 143,484 potentially defective inserts were implanted in the U.S. This figure illustrates that tens of thousands of Americans were potentially placed at risk of early implant failure. The extensive volume of affected devices and the severe health consequences experienced by patients underscore the gravity of these legal claims, as plaintiffs seek comprehensive compensation for their substantial damages, which include medical expenses, pain and suffering, and lost wages incurred due to the defective implants.
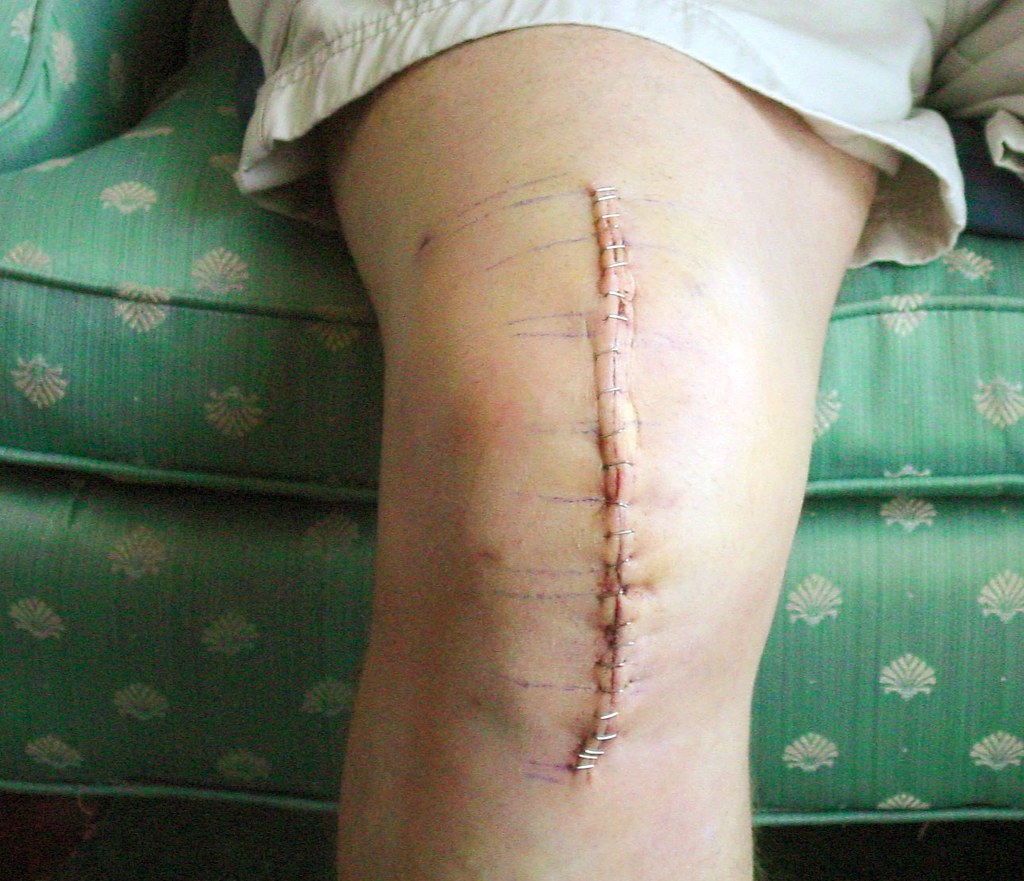
10. **Exactech’s Troubled History: The Optetrak Knee and the ‘Silent Recall’**Exactech’s history of issues with its Optetrak knee replacement implant system extends well beyond the recent widespread recalls linked to packaging defects. The Optetrak device gained market access through the abbreviated 510(k) FDA clearance process. This system permits manufacturers to market a device if it is deemed “substantially equivalent” to a device approved prior to 1976, critically, without the requirement of extensive clinical testing. The provided context clearly states that this 510(k) process “does not equate to FDA approval” and inherently “comes with risk.” Indeed, very soon after the Optetrak implant’s release, Exactech reportedly became aware of abnormally high failure rates associated with the system when compared to other knee replacement devices available on the market.
Further evidence of these early problems emerged through reports submitted to the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database. These reports indicated that Optetrak knee implants were failing at a significantly higher rate than other types of implant devices. The volume of complaints about Optetrak implants in MAUDE became so extensive that, as the context notes, a search of the database “exceeds the display limit of 500.” Compounding this, the adverse reports found in MAUDE only represented a fraction of the actual failures, as Exactech was reportedly “internally aware of countless other failures that were not reported” to the public database. This significant discrepancy between reported and internally known adverse events points to a considerable underreporting of device failures, a finding consistent with a KFF Health News investigation published in October 2023, which revealed that in hundreds of instances, the company “took years to report adverse events to a federal database that tracks device failures.”
Between 2015 and 2017, the growing number of reports detailing excessively high failure rates with Optetrak implants spurred a series of product liability lawsuits against Exactech. These earlier legal actions alleged that the high failure rate was attributable to general design defects, specifically citing the unique “finned” design feature of the original Optetrak system. Rather than issuing public warnings or initiating a transparent recall, Exactech reportedly responded by implementing what has been widely characterized as a “silent recall.” This involved gradually phasing out the finned design without any public announcement, a tactic that has raised serious ethical questions concerning the company’s transparency and commitment to patient safety.
Even after the design modification, the alarmingly high failure rates with the Optetrak implants persisted, prompting Exactech to investigate alternative explanations for the ongoing issues. This subsequent investigation ultimately led to the discovery of the critical flaw in the vacuum-seal packaging, which was allowing oxygen to prematurely degrade and malfunction the polyethylene inserts due to oxidation. This historical pattern—beginning with early, unaddressed high failure rates, followed by a delayed and discreet response, and finally culminating in the discovery of a widespread manufacturing defect—significantly reinforces the allegations of negligence and a fundamental lack of commitment to patient safety that now form the basis of thousands of lawsuits against the company. It serves as a stark reminder that a device’s initial regulatory clearance process is not a guarantee of long-term safety, and that diligent post-market surveillance and transparent reporting are absolutely paramount in the medical device industry.
11. **Who Qualifies to File an Exactech Lawsuit**For individuals contemplating legal action against Exactech, understanding the specific criteria that determine eligibility to file a lawsuit is a crucial initial step. Generally, if you have received a recalled Exactech knee, hip, or ankle implant and subsequently required corrective revision surgery, or if your healthcare provider has advised you to undergo such surgery due to complications directly attributable to the device’s failure, you may qualify. This foundational requirement centers on having received an affected implant and experiencing a demonstrable injury or the clear necessity for further medical intervention directly linked to the device’s malfunction.
The lawsuits specifically target Exactech devices manufactured between 2004 and August 2021. To confirm whether your particular implant falls within the scope of these implicated devices, it is essential to meticulously review your medical records or consult directly with your orthopedic surgeon. Should you be uncertain about the brand or specific model of your implant, a qualified product liability lawyer can provide assistance in obtaining and reviewing the necessary medical documentation. The recall encompasses various Exactech product lines, including: Acumatch Hip with Connexion GXL liner; MCS Hip with Connexion GXL liner; Novation Hip with Connexion GXL liner; specific Optetrak Knee components (such as All-polyethylene CR Tibial Components, CR Tibial Inserts, and HI-FLEX PS Tibial Inserts); Optetrak Logic Knee components (including CR Tibial Inserts and PSC Tibial Inserts); Truliant Knee components (like CR Tibial Inserts and CRC Tibial Inserts); and the Vantage Ankle Fixed-Bearing Liner Component.
Even in situations where your surgeon has not yet formally advised revision surgery, you may still be eligible to file a lawsuit if you are experiencing certain symptoms that are indicative of implant failure. These concerning symptoms include persistent or new pain, limited mobility, stiffness, an inability to bear weight on the affected joint, or radiographic evidence of osteolysis, which is bone loss around the implant. The presence of such symptoms strongly suggests that the implant may be degrading prematurely due to the packaging defect, causing significant discomfort and progressively compromising the joint’s function and overall integrity.
Exactech had previously advised doctors to contact patients who had received recalled devices. However, if you have not yet received such a notification, it is strongly recommended that you proactively reach out to your doctor’s office to schedule a follow-up appointment. Taking this proactive step can help determine the current status of your implant and establish a clear, documented record of any symptoms or concerns you may be experiencing. Such documentation is absolutely vital for any potential legal claim. Understanding these qualifying criteria is the essential first step for injured patients as they seek justice and appropriate compensation for the harm caused by these defective medical devices.
12. **Crucial Steps for Injured Patients: What to Do Next After a Recalled Implant**If you have received an Exactech joint replacement device, particularly one manufactured between 2004 and August 2021, the immediate and most crucial step is to schedule an evaluation with your doctor, even if you are not currently experiencing noticeable symptoms. The U.S. Food and Drug Administration (FDA) explicitly advises all patients with an Exactech joint replacement device to contact their healthcare provider immediately if they experience any new or worsening pain or swelling, an inability to bear weight, grinding or other unusual noises, or weakness around the implanted device. Even in asymptomatic cases, consulting your doctor can help initiate monitoring of the device’s status, as some individuals may not present symptoms even while the device is undergoing premature degradation.
During your medical evaluation, your doctor will conduct a thorough examination and may recommend diagnostic tests, such as X-rays, to assess the condition of your implant and check for signs of wear, failure, or bone loss. Based on these clinical and diagnostic findings, your healthcare provider can advise you on the most appropriate course of action, which might range from continued vigilant monitoring to, if the implant is causing significant problems or has failed, a recommendation for revision surgery. It is of paramount importance to meticulously document all medical appointments, diagnostic test results, and prescribed treatment plans, as these records will serve as critical evidence should you decide to pursue a legal claim.
Exactech has publicly stated its commitment to patient well-being and has indicated that it has “provided substantial out-of-pocket patient reimbursements and surgeon support for related expenses.” While such offers might appear to be a straightforward solution, it is absolutely imperative to exercise extreme caution before accepting any direct compensation or signing any paperwork originating from Exactech. Agreeing to a reimbursement program or signing certain documents could inadvertently compel you to waive your legal right to file a lawsuit against the company. This action might significantly limit your ability to recover full and fair compensation for all your damages, including pain and suffering, future medical expenses, and any lost wages.
Before engaging in any direct discussions or accepting any offers from Exactech, it is strongly and unequivocally advised to consult with a qualified product liability lawyer to understand your legal rights. Many reputable attorneys typically offer free case evaluations, with no obligation, for individuals who have been injured by Exactech knee, hip, or ankle implants. An experienced attorney can provide invaluable guidance through the complexities of the situation, accurately evaluate the merits of your potential claim, and navigate you through the intricate legal process, ensuring that your rights are protected and that you receive equitable compensation for the injuries and losses you have endured. Furthermore, they can assist in verifying your implant’s recall status and in gathering all necessary medical evidence to support your case.

13. **Bankruptcy’s Shadow: Impact on Exactech Lawsuit Settlements and Compensation**Exactech’s decision to file for Chapter 11 bankruptcy protection in October 2024 has fundamentally reshaped the legal landscape for thousands of ongoing lawsuits and the prospects of potential settlements. This significant corporate action triggered an “automatic stay,” which effectively paused nearly all litigation against the company, including the federal MDL proceedings and numerous state court cases. The primary objective of a Chapter 11 bankruptcy for a company like Exactech is to restructure its substantial debt and operational framework, a process that typically involves a complex mechanism for addressing creditor claims, including those from injured patients and other legal claimants.
The bankruptcy filing has directly impacted the initial projections for Exactech lawsuit settlements, leading to revised expectations. Previously, legal experts estimated potential Exactech lawsuit settlements to range between $100,000 and $300,000, a projection based on settlement patterns observed in past knee replacement product liability lawsuits. However, the available context explicitly states that “It is unlikely” that patients will still see average Exactech settlement numbers within this anticipated range, given the complexities introduced by the bankruptcy. This downward adjustment in expectations stems from the fact that the bankruptcy process typically necessitates the establishment of a compensation trust to disburse payments for claims against the company. The funding for such a trust will be ultimately determined by Exactech’s remaining assets and requires the stringent approval of the bankruptcy court. Consequently, “the settlement values for Exactech knee implant lawsuits may be significantly lower than the settlement range we initially expected.”
In a crucial development for claimants, a revised bankruptcy exit plan, confirmed in September 2025, stipulated that Exactech’s original private equity backer would no longer be shielded from liability. Instead, a dedicated trust will be created specifically to handle injury payouts to affected patients. The confirmation hearing for this plan was reportedly pushed to late June, underscoring the intricate and often protracted negotiations involved in securing adequate funding and establishing the framework for this trust. Creditor estimates have suggested that potential injury claims could collectively exceed $1 billion, a figure that dramatically contrasts with the company’s initial proposal of only $10 million, highlighting the immense financial implications and the ongoing battle for fair and just compensation.
As of June 2025, there have been no global settlements reached in the Exactech lawsuits. While the bankruptcy filing undoubtedly introduces a substantial layer of uncertainty regarding the ultimate payout values for individual claimants, legal experts maintain a position of “cautious optimism” about securing “reasonable Exactech settlement amounts that closely approximate their true value.” The bankruptcy court is anticipated to establish a clear and structured process for addressing claims, and critically, new claims must now be filed directly through the bankruptcy court system. While the “full value” of individual cases might be subject to compromise under the bankruptcy proceedings, the establishment of a claimant trust is designed to ensure that injured parties do, in fact, receive some form of compensation as Exactech endeavors to move forward under its new ownership structure.
14. **Exactech’s Restructuring and New Chapter: A Look at the Company’s Future Under New Ownership**
In a pivotal development, Exactech announced on September 15, 2025, the Court’s confirmation of its restructuring plan and the approval of the sale of its assets to a new ownership group. This milestone signifies a critical turning point for the company, marking its transition “past the restructuring process,” with the customary closing steps for the sale anticipated to be completed in the ensuing weeks. The new ownership consortium comprises Strategic Value Partners, LLC (SVP), Stellex Capital Management LLC, and Greywolf Capital Management LP, who will acquire substantially all of Exactech’s operations and assets, thereby ushering in a new era for the medical technology firm.
Despite the formidable challenges posed by the bankruptcy and widespread recalls, Exactech maintained its operational status throughout the proceedings, ensuring continuity for its existing product lines and services. The approved sale is strategically designed to position the newly formed company to fulfill its core mission: “to be the leading surgeon partner in orthopedics.” Key product lines, which include the highly regarded Equinoxe® Shoulder; Vantage Ankle®; Alteon® and Spartan Hip; Truliant®, Triverse® and Newton® Knee systems; and GPS navigation technologies, are slated to transfer to the new company. This transition also encompasses Exactech’s deep engineering and manufacturing expertise, alongside the extensive product data amassed over four decades, indicating a clear intent to leverage its historical strengths while rigorously addressing and rectifying past product failures.
The revitalized company will be steered by its incoming Chief Executive Officer, Aurelio Sahagun, and overseen by a new Board of Directors, which will provide crucial oversight of the new company’s strategy and governance. Clara Anderson, Managing Director at SVP, expressed enthusiasm about the new entity’s commitment to a “quality imperative, its commitment to innovation, and its goal of being the most trusted partner to orthopedic surgeons.” This statement from the new ownership unequivocally signals a clear intent to prioritize product quality and to diligently rebuild the trust that was significantly eroded due to severe scrutiny over previous product defects. Furthermore, the new owners have articulated plans to “continue to invest in the new company and accelerate opportunities to drive innovation and growth,” aiming to regain market confidence.
Exactech’s comprehensive restructuring and the introduction of new ownership unequivocally mark the beginning of a new chapter, one aimed at fostering long-term growth and sustained success. While the complex legal battles and the crucial patient compensation process continue to unfold under the established bankruptcy trust, the company is actively striving to redefine its future trajectory. This transformative transition, bolstered by substantial private equity investment, represents an intricate and determined effort to stabilize a prominent medical technology leader and move decisively beyond the profound challenges posed by widespread product recalls and the ensuing litigation. For patients and the broader medical device industry alike, this evolution underscores the continuous, unwavering need for stringent safety standards, transparent reporting, and robust accountability to ensure that medical advancements genuinely enhance, rather than compromise, patient well-being. The collective hope is that under this renewed leadership and strategic direction, Exactech can genuinely embody its stated commitment to patient care and pioneering innovation, restoring its reputation as a trusted partner in orthopedic health.
The saga of Exactech’s defective implants serves as a stark reminder of the critical importance of unwavering vigilance in the medical device industry. While the complex legal and financial ramifications continue to unfold, with thousands of patients seeking justice and the company navigating a multifaceted bankruptcy, the core message remains undeniably clear: patient safety must always be paramount. For those affected, staying meticulously informed, seeking appropriate medical and legal counsel, and diligently advocating for their rights are absolutely essential steps on the arduous path to recovery and accountability. As Exactech embarks on this new chapter under fresh ownership, the industry, regulatory bodies, and patients alike will be watching closely to ensure that the profound lessons learned from this challenging period lead to enduring improvements in product integrity, transparent practices, and steadfast corporate responsibility. Our collective commitment, as consumers and patient advocates, is to continue pushing relentlessly for the highest standards of safety and transparency, ensuring that the sacred trust placed in medical advancements is never again betrayed.”
, “_words_section2”: “1964



