In an era defined by an insatiable quest for innovative energy solutions and a deeper understanding of the universe’s foundational elements, hydrogen stands out as a subject of profound and enduring fascination. Far from a mere entry on the periodic table, this element, with its deceptively simple atomic structure, holds secrets that stretch from the dawn of time to the potential powering of tomorrow’s world.
Indeed, hydrogen is not just the lightest element; it is the most abundant chemical element in the universe, a silent architect of stars and a fundamental component of the cosmos. Its journey through scientific discovery has illuminated principles of quantum mechanics, unveiled a spectrum of isotopes with distinct applications, and challenged our understanding of matter under extreme conditions. The insights gleaned from studying hydrogen offer a panoramic view of chemistry, physics, and astrophysics.
As we navigate the complexities of our technological future, a comprehensive grasp of hydrogen’s intrinsic properties and behaviors becomes increasingly vital. This deep dive into its essential facts serves as a bedrock for understanding its emerging significance, illuminating the pathways that scientists and innovators are exploring to harness its potential. Let us embark on an exploration of the fundamental truths about hydrogen, laying the groundwork for appreciating its transformative role in the grand tapestry of existence and innovation.

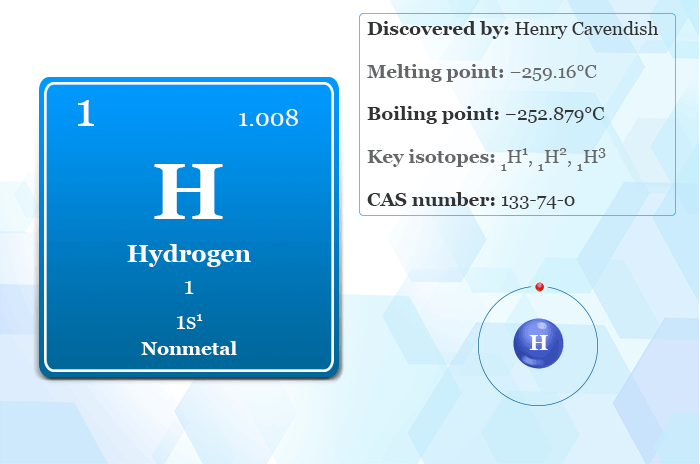
1. **Hydrogen’s Fundamental Nature: The Universe’s Prime Element**Hydogen, denoted by the symbol H and atomic number 1, is more than just another chemical element; it is the lightest and most abundant chemical element in the universe. Astonishingly, it constitutes about 75% of all normal matter, making it the fundamental building block from which everything else is forged. This pervasive presence underscores its pivotal role in cosmic evolution and the formation of celestial bodies.
Under standard conditions, hydrogen exists as a gas of diatomic molecules, commonly referred to as dihydrogen, or sometimes simply hydrogen gas, molecular hydrogen. This H2 molecule is characterized by its colorless, odorless, non-toxic, and highly combustible nature. These characteristics define its behavior in various terrestrial and extraterrestrial environments, influencing everything from atmospheric chemistry to industrial applications.
Stars, including our own Sun, are colossal furnaces where hydrogen predominantly exists in a plasma state, undergoing nuclear fusion to generate light and heat. On Earth, while elemental H2 gas is present, hydrogen is primarily found bound within molecules, most notably in water (H2O) and a vast array of organic compounds essential for life. The most common isotope of hydrogen, 1H, is remarkable for its simplicity, consisting of a single proton and one electron, devoid of neutrons.
Its unique electron configuration, 1s1, allows it to readily form covalent bonds with most nonmetals, contributing to the formation of ubiquitous compounds like water and various organic substances. This versatility in bonding underscores its central role in chemical reactions, particularly acid-base dynamics, which fundamentally involve the exchange of protons among soluble molecules. In ionic compounds, hydrogen can manifest as either a negatively-charged anion, known as hydride, or a positively-charged cation, H+, which is often referred to as a proton.
While hydride is rarely observed due to its tendency to deprotonate solvents, the H+ cation, though tightly bonded to water molecules in aqueous solutions, profoundly influences their behavior, a fact reflected in the critical importance of pH. This duality in its chemical behavior, coupled with its cosmic abundance, cements hydrogen’s status as an indispensable element for understanding both the macroscopic universe and the microscopic world of chemical interactions.

2. **The Genesis of Understanding: Hydrogen’s Discovery and Naming**The journey to identifying hydrogen as a distinct element is a fascinating chapter in the history of chemistry, beginning in the 17th century with rudimentary observations. It was in 1671 that the Irish scientist Robert Boyle first described the reaction between iron filings and dilute acids, a process that notably resulted in the production of hydrogen gas. Although Boyle did not initially recognize the gas’s flammability, his observations laid early groundwork, inadvertently setting the stage for later breakthroughs that would challenge and ultimately overturn the prevailing phlogiston theory of combustion.
Nearly a century later, a more definitive recognition emerged from the meticulous work of Henry Cavendish. Between 1766 and 1781, Cavendish not only identified this gas as a distinct substance, which he termed “inflammable air,” but also made a crucial discovery: it produced water when burned. This property was so fundamental that he is widely credited with the discovery of hydrogen as an element. Cavendish even speculated that his “inflammable air” was identical to the hypothetical substance “phlogiston,” highlighting the scientific paradigms of his time.
The final piece of the naming puzzle was put into place by Antoine Lavoisier in 1783. Lavoisier, working with Laplace, successfully reproduced Cavendish’s finding that water is produced when hydrogen burns, solidifying the understanding of this elemental reaction. It was Lavoisier who identified the element and, drawing from Ancient Greek, named it “hydrogen,” meaning ‘water-former’ (from ὕδωρ, romanized: húdōr, lit. ‘water’, and γεννάω, gennáō, ‘I bring forth’). This name perfectly encapsulated its defining chemical property.
Lavoisier’s experiments were often designed to study mass conservation, and to produce hydrogen for his investigations, he employed a method of treating metallic iron with a stream of water through an incandescent iron tube heated in a fire. This anaerobic oxidation of iron by water’s protons at high temperature is schematically represented by reactions such as Fe + H2O → FeO + H2, or 3 Fe + 4 H2O → Fe3O4 + 4 H2. While this method was crucial for historical discovery, it’s worth noting that similar H2-producing processes can be problematic in modern contexts, such as the corrosion of zirconium cladding on nuclear fuel rods, demonstrating the element’s dual nature in both scientific advancement and engineering challenges.
3. **Hydrogen’s Quantum Leap: Unraveling Atomic Structure**Hydrogen’s incredibly simple atomic structure, consisting of just a single proton and one electron, has positioned it at the very heart of theoretical physics and the development of quantum mechanics. Its straightforward composition makes it an ideal model system for understanding fundamental principles, profoundly influencing our comprehension of atomic structure and the behavior of light.
The interaction of light with hydrogen atoms, specifically the spectrum of light they emit or absorb, became a cornerstone in the early 20th century. The energy levels of a hydrogen atom, for instance, can be calculated with remarkable accuracy using the Bohr model. In this classical yet quantum-inspired framework, the electron is imagined to “orbit” the proton, akin to a planet circling a star, though the forces are electrostatic attraction rather than gravity. Crucially, early quantum mechanics, through Bohr’s postulation of angular momentum discretization, dictated that the electron could only occupy specific, allowed distances from the proton, and thus only certain discrete energy levels. The ground state energy level of the electron in a hydrogen atom is a precise −13.6 electronvolts (eV), a value equivalent to an ultraviolet photon of roughly 91 nanometers wavelength.
These quantized energy levels are referred to by consecutive quantum numbers, with n = 1 representing the ground state. The mesmerizing hydrogen spectral series corresponds precisely to the emission of light as electrons transition from higher to lower energy levels, providing empirical validation for quantum theory. Adding another layer of complexity, each of these energy levels is further subtly split into four hyperfine levels due to intricate spin interactions between the electron and the proton, revealing the atom’s internal magnetic dynamics.
For high-precision definitions of physical constants, the exact values for hydrogen atom energy levels are absolutely critical. Quantum calculations have meticulously identified nine distinct contributions to these energy levels, with the eigenvalue derived from the Dirac equation providing the largest and most significant input. Other essential terms, such as relativistic recoil, the self-energy, and the vacuum polarization terms, further refine these calculations. Hydrogen’s unparalleled status as the only neutral atom for which the Schrödinger equation can be directly solved has been instrumental in deepening our understanding of quantum mechanics. Moreover, the study of the corresponding simplicity of the hydrogen molecule (H2) and its cation (H+2) shortly after the mid-1920s quantum mechanical treatment of the hydrogen atom had been developed, ushered in profound insights into the very nature of the chemical bond, forever transforming our view of molecular interactions.
4. **The Diverse World of Hydrogen Isotopes: Protium, Deuterium, and Tritium**One of the most compelling aspects of hydrogen is its set of naturally occurring isotopes, each possessing unique characteristics and applications. Hydrogen stands out among the elements for assigning distinct, commonly used names to its isotopes, a legacy from the early study of radioactivity where heavy radioisotopes were often given their own designations. While 2H and 3H are preferred by the International Union of Pure and Applied Chemistry (IUPAC), the symbols D for deuterium and T for tritium are frequently encountered.
The most common hydrogen isotope is 1H, known descriptively but rarely formally as protium, making up over 99.98% of natural hydrogen. Its nucleus is uniquely simple, consisting solely of a single proton with no neutrons, a feature that distinguishes it as the only stable isotope without neutrons. This simplicity contributes to its fundamental role in atomic physics and chemistry.
The second stable isotope, 2H, is called deuterium, and its nucleus contains one proton and one neutron. Scientists believe nearly all deuterium nuclei in the universe originated during Big Bang nucleosynthesis and have persisted since. Deuterium is not radioactive, nor does it pose a significant toxicity hazard, making it valuable in various scientific and industrial contexts. Water enriched with molecules containing deuterium instead of normal hydrogen is famously known as heavy water. Deuterium and its compounds serve as non-radioactive labels in chemical experiments and as solvents in 1H-NMR spectroscopy, a powerful analytical technique. Furthermore, heavy water is critical as a neutron moderator and coolant in certain nuclear reactors, and deuterium itself is recognized as a potential fuel for commercial nuclear fusion, holding immense promise for future energy generation.
Finally, 3H, or tritium, is the radioactive isotope of hydrogen, featuring one proton and two neutrons in its nucleus. Tritium undergoes beta decay, transforming into helium-3 with a half-life of 12.32 years. Despite its radioactivity, its decay is gentle enough for practical applications. For instance, it’s used in luminous paint to enhance the visibility of data displays, such as the hands and dial-markers of watches, with the watch glass safely preventing the small amount of radiation from escaping. Trace amounts of tritium are naturally produced by cosmic rays interacting with atmospheric gases, and it has also been released historically in nuclear weapons tests. Beyond luminous applications, tritium finds use in nuclear fusion research, as a tracer in isotope geochemistry, and in specialized self-powered lighting devices, further illustrating the diverse utility derived from hydrogen’s isotopic variations. The existence of antihydrogen, the antimatter counterpart consisting of an antiproton and a positron, and exotic atoms like muonium, further expands the fascinating frontier of hydrogen’s atomic identity.

5. **Dihydrogen (H2) and Its Volatile Nature: A Force to Be Reckoned With**Under standard conditions, hydrogen exists predominantly as a gas of diatomic molecules, referred to officially as “dihydrogen.” However, it’s commonly known as “molecular hydrogen” or simply hydrogen. This form of hydrogen is distinct in its physical attributes: it is a colorless, odorless, and highly flammable gas, characteristics that dictate both its utility and its inherent risks across a spectrum of applications. Its flammability, in particular, has shaped its historical uses and the safety protocols surrounding its handling.
One of the most significant chemical properties of dihydrogen is its combustion. Hydrogen gas is exceptionally flammable, reacting vigorously with oxygen in the air to produce liquid water. This reaction, represented by the equation 2 H2(g) + O2(g) → 2 H2O(l), is highly exothermic. The amount of heat released is substantial, measuring −286 kilojoules (kJ) per mole of hydrogen, or an impressive 141.865 megajoules (MJ) for a one-kilogram mass. This energetic release makes hydrogen an attractive, albeit challenging, candidate for various energy conversion technologies.
However, this high reactivity also presents considerable hazards. Hydrogen gas forms explosive mixtures with air over a wide range of concentrations, from 4% to 74%, and with chlorine from 5% to 95%. The hydrogen autoignition temperature, the point at which spontaneous ignition occurs in air, is 500 °C (932 °F). A particularly dangerous scenario arises in a high-pressure hydrogen leak, where the shock wave generated by the leak itself can compress and heat the surrounding air sufficiently to reach the autoignition temperature, potentially leading to immediate flaming and even an explosion. Such incidents underscore the critical need for sophisticated detection and safety measures.
Compounding the danger, hydrogen flames are notoriously difficult to detect with the eye in daylight, as they emit only faint blue and ultraviolet light. This near invisibility necessitates the use of specialized flame detectors in any environment where hydrogen is handled or stored, ensuring that fires can be identified and addressed promptly to prevent catastrophic outcomes. Despite these challenges, its powerful combustion makes it a compelling option for future energy systems, provided safety can be assured.
Historically, hydrogen’s low density—only 7% that of air—made it a prime candidate as a lifting gas for balloons and airships. The first hydrogen-filled balloon was invented by Jacques Charles in 1783, and Henri Giffard’s hydrogen-lifted airship in 1852 marked the advent of reliable air travel. German count Ferdinand von Zeppelin later popularized rigid airships, the Zeppelins, which saw their maiden flight in 1900 and carried thousands of passengers without incident until 1914. Hydrogen-lifted blimps even served as observation platforms and bombers during World War II. However, the tragic Hindenburg disaster in 1937, where the hydrogen filling the airship ignited, possibly by static electricity, led to the cessation of commercial hydrogen airship travel. Today, hydrogen is still used, often in preference to more expensive helium, as a lifting gas for meteorological weather balloons, highlighting its enduring, albeit cautious, application in atmospheric science.
_-_Museo_scienza_e_tecnologia_Milano.jpg)
6. **The Enigma of Hydrogen’s Spin Isomers: Ortho- and Para-hydrogen**One of the more intricate and fascinating aspects of molecular hydrogen (H2) lies in the existence of its two nuclear spin isomers: orthohydrogen and parahydrogen. These isomers are not distinct chemical compounds but rather represent different quantum states of the same molecule, distinguished solely by the relative orientations of the spins of their nuclei. In the orthohydrogen form, the spins of the two hydrogen nuclei are parallel, resulting in a spin triplet state with a total molecular spin S = 1. Conversely, in the parahydrogen form, the nuclear spins are antiparallel, leading to a spin singlet state with a total spin S = 0. This subtle difference in spin configuration has profound implications for the molecule’s thermal and physical properties.
The equilibrium ratio of orthohydrogen to parahydrogen is temperature-dependent. At room temperature or warmer, equilibrium hydrogen gas typically consists of approximately 25% parahydrogen and 75% orthohydrogen. However, orthohydrogen is an excited state, possessing a higher energy than the parahydrogen form by a measurable 1.455 kJ/mol. When cooled to low temperatures, orthohydrogen will slowly convert to the lower-energy parahydrogen form over several minutes, a process that is critical in certain industrial applications. The distinct rotational quantum states of these isomers mean their thermal properties differ significantly, impacting how they absorb and release energy.
The ortho-to-para ratio in H2 is a particularly important consideration in the liquefaction and subsequent storage of liquid hydrogen. The conversion from orthohydrogen to parahydrogen is an exothermic process, meaning it releases heat. If this conversion occurs unchecked during the cooling and storage process, the heat generated can be substantial enough to cause a significant portion, or even most, of the liquid hydrogen to evaporate. This undesirable loss of precious liquid hydrogen necessitates careful management during its production and storage. To circumvent this issue, catalysts that promote the ortho-para interconversion, such as ferric oxide and activated carbon compounds, are strategically employed during the hydrogen cooling process. These catalysts accelerate the conversion to the more stable parahydrogen form at cryogenic temperatures, thereby minimizing heat release and preventing substantial liquid hydrogen loss. This sophisticated management of spin isomers underscores the advanced engineering required for efficient hydrogen handling and storage.
Interestingly, the existence and peculiar behavior of these spin isomers were among the first quantum effects to be explicitly noticed, long before they were fully understood. James Clerk Maxwell, for instance, observed that the specific heat capacity of H2 inexplicably deviated from that expected of a diatomic gas below room temperature, progressively resembling that of a monatomic gas at cryogenic temperatures. Quantum theory later elucidated that this anomalous behavior arises from the wide spacing of the quantized rotational energy levels in H2, a consequence of its exceptionally low mass. These widely spaced levels effectively inhibit the equal partition of heat energy into rotational motion at low temperatures, a phenomenon not observed in diatomic gases composed of heavier atoms where the rotational levels are much more closely spaced. This historical observation highlights how hydrogen has consistently served as a frontier for probing the fundamental laws of physics.

7. **Hydrogen’s Extreme Phases: From Liquid to Metallic Plasma**Beyond its familiar gaseous state, hydrogen exhibits a remarkable array of phases under various extreme conditions of temperature and pressure, each revealing fascinating physical properties and behaviors. These diverse phases range from cryogenic liquids and solids to exotic metallic and plasma states, offering a glimpse into hydrogen’s versatility across vast cosmic and laboratory environments.
Liquid hydrogen, a crucial form for storage and transport in many emerging energy applications, can exist at temperatures below its critical point of 33 kelvins (−240.2 °C; −400.3 °F). However, to achieve a fully liquid state at atmospheric pressure, H2 must be cooled even further, to an intensely frigid 20.28 K (−252.87 °C; −423.17 °F). This achievement was a monumental scientific feat, first accomplished by James Dewar in 1898. Dewar leveraged both regenerative cooling techniques and his groundbreaking invention, the vacuum flask, to successfully liquefy hydrogen, paving the way for low-temperature physics and engineering.
Pushing the temperatures even lower, liquid hydrogen transitions into solid hydrogen at standard pressure below its melting point of 14.01 K (−259.14 °C; −434.45 °F). Intriguingly, solid hydrogen is not a monolithic state; distinct solid phases, designated Phase I through Phase V, have been identified. Each of these phases is characterized by a unique molecular arrangement and crystal structure, reflecting the complex interplay of quantum mechanical forces at extreme cryogenic temperatures. For instance, at its triple point, liquid and solid phases can coexist in a mixture known as slush hydrogen, offering specific advantages in terms of density and heat absorption for propellant applications.
Perhaps the most exotic and mind-bending phase of hydrogen is metallic hydrogen. This state is achieved under incredibly immense pressures, exceeding 400 million Pascals (58,000 psi). In this phase, hydrogen is believed to transform into an electrical conductor, behaving much like a metal. While challenging to produce and study on Earth, metallic hydrogen is theorized to exist deep within the cores of giant planets like Jupiter, contributing to their powerful magnetic fields and internal dynamics. The prospect of terrestrial metallic hydrogen excites researchers for its potential as a superconductor or a highly efficient rocket propellant.
Completing hydrogen’s phase spectrum, when ionized, hydrogen transcends into a plasma state. In plasma, electrons are stripped from the atoms, creating a hot, ionized gas. This is the predominant form in which hydrogen exists within stars, where temperatures and pressures are so extreme that atoms cannot maintain their neutral structure. Stellar plasma is the fuel for nuclear fusion, the process that powers stars and provides the energy for the universe. The thermal and physical properties of hydrogen, from its density and specific heat to its viscosity and thermal conductivity, fluctuate dramatically across these various phases and temperatures, as comprehensively detailed in extensive tables mapping its behavior from 100 K to 2000 K. Understanding these extreme phases is not merely an academic exercise; it is crucial for astrophysical modeling, advanced materials science, and the ambitious pursuit of controlled nuclear fusion.
Navigating from hydrogen’s foundational attributes, we now journey to its expansive presence throughout the cosmos and right here on Earth, before delving into the cutting-edge methods being developed to bring this versatile element into our sustainable energy future. Understanding where hydrogen exists and how we can harness it is paramount to unlocking its full potential as a game-changer in the global energy landscape.

8. **Hydrogen’s Cosmic Abundance: The Fabric of the Universe**Hydrogen, in its atomic form (H), reigns supreme as the most abundant chemical element in the universe, making up an astonishing 75% of all normal matter by mass and over 90% by the sheer number of atoms. This incredible ubiquity highlights its foundational role in the universe’s formation and evolution. In the very early universe, protons rapidly formed within the first second after the Big Bang, eventually coalescing into neutral hydrogen atoms approximately 370,000 years later during what scientists call the recombination epoch. This critical period marked a significant expansion and cooling of the primordial plasma, finally allowing electrons to become stably bound to protons.
In the vast realms of astrophysics, neutral hydrogen within the interstellar medium is designated as H I, while its ionized counterpart is known as H II. The powerful radiation emanating from nascent stars strips electrons from H I atoms, thus creating expansive spheres of ionized H II around these stellar nurseries. The chronology of the universe reveals that neutral hydrogen predominated until the birth of the first stars, ushering in an era of reionization. This process produced growing bubbles of ionized hydrogen that gradually merged over hundreds of millions of years, a phenomenon actively probed by detecting the distinctive 21-centimeter hydrogen line at 1420 MHz, offering invaluable insights into primordial hydrogen.
Hydrogen is also found in immense abundance within stars themselves and across the colossal gas giant planets. Intricate molecular clouds of H2 are intricately linked with the processes of star formation, acting as stellar nurseries where new suns are born. Moreover, hydrogen plays an absolutely vital role in powering stars, fueling them through the proton-proton reaction in lower-mass stars, much like our own Sun, and through the more complex CNO (Carbon-Nitrogen-Oxygen) cycle of nuclear fusion in stars that are significantly more massive. This continuous stellar fusion underscores hydrogen’s ceaseless contribution to the universe’s energy output.
Beyond neutral and ionized forms, protonated molecular hydrogen (H+3) is a surprisingly common sight in the interstellar medium, where it is primarily generated through the ionization of molecular hydrogen by cosmic rays. This robust ion has also been observed in the upper atmosphere of Jupiter, demonstrating its prevalence even within our solar system. Due to the extremely low temperatures and densities characteristic of outer space, H+3 is remarkably long-lived, playing a notable and active role in shaping the complex chemistry of the interstellar medium. Intriguingly, its neutral cousin, triatomic hydrogen (H3), can only exist in an excited and inherently unstable form, further emphasizing the unique stability and importance of the protonated version.
9. **Terrestrial Hydrogen: Abundance in Compound Forms**While a colossal constituent of the universe, hydrogen’s presence on Earth’s surface takes on a different character. It stands as the third most abundant element on our planet’s crust, though it primarily exists within a myriad of chemical compounds rather than as a free element. Its widespread integration into substances such as hydrocarbons, which form the bedrock of organic chemistry and fossil fuels, and most notably water (H2O), means that hydrogen is literally all around us, albeit in bound forms crucial for life itself and industrial processes.
Elemental hydrogen, in its gaseous H2 form, is typically found at very low concentrations within Earth’s atmosphere, averaging around 0.53 parts per million on a molar basis. This scarcity of free atmospheric H2 is primarily attributed to its incredibly light weight. Being the lightest element, hydrogen molecules can achieve escape velocity more readily than heavier gases, allowing them to escape Earth’s gravitational pull and dissipate into space over time. This continuous escape mechanism prevents significant accumulation of free hydrogen gas in our planet’s air envelope.
Despite its relatively low atmospheric concentration, terrestrial hydrogen is sufficiently abundant to play a crucial role in the metabolism of several varieties of bacteria, highlighting its ongoing participation in Earth’s intricate biochemical cycles. In a development that could profoundly reshape our energy future, large underground deposits of hydrogen gas have recently been discovered in various countries, including Mali, France, and Australia. However, as of 2024, the economic viability and scalability of extracting these vast subterranean hydrogen reserves remain uncertain, posing a significant challenge that scientists and engineers are actively working to overcome. These potential natural stores represent a tantalizing prospect for sustainable energy, if extraction proves feasible.
10. **Industrial Hydrogen Production: The Reign of Steam Methane Reforming**Currently, nearly all of the world’s supply of hydrogen gas (H2) for industrial applications is derived from fossil fuels, a fact that underscores the urgency for developing more sustainable production methods. While a multitude of techniques exist for generating H2, three processes predominantly dominate commercial production: steam reforming, often meticulously coupled with the water-gas shift reaction; partial oxidation of hydrocarbons; and water electrolysis. These methods, while effective, each carry distinct implications for energy consumption and environmental impact.
Among these, steam methane reforming (SMR) stands out as the primary method, revolving around the reaction of water and methane. This high-temperature process, typically conducted between 1,000–1,400 K (730–1,130 °C), involves steam (water vapor) reacting with methane to yield a mixture of carbon monoxide and hydrogen (CO + 3 H2). The significant drawback of this widely adopted method is its considerable carbon footprint, as producing just one tonne of hydrogen through SMR emits between 6.6 and 9.3 tonnes of carbon dioxide, not including additional emissions like vented and fugitive methane from the natural gas feedstock.
Although the core SMR reaction is thermodynamically favored at lower pressures, industrial operations are typically conducted at high pressures, around 2.0 MPa (20 atm). This choice is driven by two key factors: high-pressure H2 is the most marketable product, simplifying storage and distribution, and pressure swing adsorption (PSA) purification systems, essential for achieving high-purity hydrogen, operate much more efficiently at elevated pressures. The resulting product mixture is famously known as “synthesis gas,” due to its versatile application as a direct feedstock for the synthesis of methanol and a wide array of other crucial chemical compounds.
One of the persistent complications in this highly optimized technology is the unwanted formation of coke, or solid carbon, represented by the reaction CH4 → C + 2 H2. To mitigate this issue, steam reforming processes typically employ an excess of H2O. Furthermore, additional hydrogen can be effectively recovered from the synthesis gas by utilizing the carbon monoxide generated through the water gas shift (WGS) reaction (CO + H2O → CO2 + H2), a process that requires the presence of an iron oxide catalyst. It is also worth noting that in certain industrial contexts, such as the Haber process for ammonia production, hydrogen is generated and consumed within the same integrated system, eliminating the need for separate storage or transportation.
11. **Alternative Industrial Pathways: Partial Oxidation and Water Electrolysis**Beyond the dominant steam methane reforming, other industrial avenues contribute to hydrogen production, each with its own advantages and challenges. Partial oxidation of hydrocarbons offers another route for generating carbon monoxide and hydrogen. This process, exemplified by the reaction 2 CH4 + O2 → 2 CO + 4 H2, is generally considered less commercially significant than SMR for dedicated hydrogen production. However, it’s a viable option, and historical methods also demonstrate that coal can serve as a precursor to the water-gas shift reaction (C + H2O → CO + H2). Additionally, olefin production units, particularly those involved in cracking light feedstocks like ethane or propane, often produce substantial quantities of byproduct hydrogen, which can then be captured and utilized.
A conceptually straightforward and increasingly vital method is the electrolysis of water. This elegant process involves passing an electric current through water to decompose it into its constituent elements: 2 H2O(l) → 2 H2(g) + O2(g). Commercial electrolyzers typically employ nickel-based catalysts within strongly alkaline solutions to facilitate this reaction efficiently. While platinum is recognized as an even superior catalyst, its high cost often limits its widespread industrial application. A significant environmental advantage of electrolysis is that when the electricity used is sourced from renewable energy—such as solar, wind, or hydropower—the hydrogen produced is commonly referred to as “green hydrogen,” representing a truly clean energy carrier.
Electrolysis also plays a dual role in other chemical industries. For instance, the electrolysis of brine, primarily conducted to yield chlorine, coincidentally produces high-purity hydrogen as a co-product. This hydrogen is then often harnessed for a variety of subsequent chemical transformations, including crucial hydrogenation reactions. Despite its environmental appeal when powered by renewables, the electrolysis process, at current scales and without significant technological advancements, generally remains more expensive than producing hydrogen from methane, particularly if carbon capture and storage technologies are not factored into the fossil fuel-based methods.
The path toward more widespread adoption of green hydrogen, however, is heavily reliant on technological innovation. Breakthroughs in hydrogen electrolyzers have the potential to dramatically reduce production costs, making large-scale generation of hydrogen from electricity significantly more cost-competitive. As research and development continue to advance, these innovations could unlock a future where water electrolysis becomes a primary, economically viable source of clean hydrogen, fundamentally transforming the energy landscape by decoupling hydrogen production from fossil fuel dependency.
12. **Methane Pyrolysis: A Lower-Carbon Hydrogen Production Approach**Methane pyrolysis presents a promising, albeit still developing, industrial route for hydrogen production that offers a potentially lower carbon footprint compared to traditional steam methane reforming. This process involves the thermal decomposition of natural gas (methane) in the absence of oxygen, aided by a catalyst, to produce hydrogen gas and solid carbon. The fundamental reaction can be simply represented as CH4(g) → C(s) + 2 H2(g), with an associated input heat (ΔH°) of 74 kJ/mol required to drive the decomposition.
Unlike SMR, which produces carbon dioxide as a gaseous byproduct, methane pyrolysis yields solid carbon. This solid carbon product is a key differentiator, as it can be directly sold as a valuable manufacturing feedstock, used as a fuel, or safely landfilled, offering various downstream utilization pathways that avoid direct atmospheric emissions of CO2. This characteristic is what gives methane pyrolysis its potential for a significantly lower carbon footprint than many existing hydrogen production processes, particularly those that do not incorporate carbon capture and storage technologies.
While the concept is compelling, there are significant hurdles to overcome before methane pyrolysis can achieve widespread industrial-scale adoption. Paramount among these are the engineering challenges associated with effectively removing the solid carbon from the reaction environment. The accumulation of carbon can foul and deactivate the catalysts crucial for the process, severely hindering efficiency and requiring frequent regeneration or replacement. Developing robust, continuous mechanisms for carbon removal and preventing its undesirable reaction with the catalyst are active areas of research.
Despite these obstacles, the allure of producing hydrogen without generating gaseous CO2 emissions directly makes methane pyrolysis a strategically important area of innovation. Continued research and technological advancements in catalyst design, reactor engineering, and carbon management are essential to unlock the full potential of this method. If these challenges can be effectively addressed, methane pyrolysis could become a vital component in a diversified portfolio of sustainable hydrogen production technologies, helping to accelerate the transition to a cleaner energy economy.

13. **Biohydrogen: Harnessing Nature’s Own Production Mechanisms**Moving beyond industrial processes, nature itself offers fascinating pathways for hydrogen production, primarily through biological systems. H2 gas is naturally produced in various organisms by a specialized class of enzymes known as hydrogenases. This remarkable enzymatic activity allows the host organisms to efficiently utilize fermentation as a primary source of energy, playing a crucial role in their metabolic processes. These biological factories demonstrate hydrogen’s inherent role in the cycle of life.
Intriguingly, these same hydrogenase enzymes possess a dual capability: they can also catalyze the oxidation of H2. This reverse reaction allows certain host organisms to subsist by reducing oxidized substrates, effectively using electrons extracted from hydrogen to power their cellular machinery. This metabolic flexibility highlights the central importance of hydrogen in diverse ecological niches, where it serves as both an energy carrier and a redox mediator, facilitating vital electron transfer processes within biological systems.
At the heart of these versatile hydrogenase enzymes are sophisticated active sites that typically feature either iron or intricate nickel-iron centers. These metal cofactors are precisely engineered by evolution to facilitate the reversible conversion between molecular hydrogen and protons plus electrons. The efficiency and specificity of these biological catalysts offer valuable insights for developing biomimetic approaches to hydrogen production, inspiring new designs for synthetic catalysts that could operate under milder, more sustainable conditions than many industrial methods.
Furthermore, biological water splitting represents a natural process that holds immense potential for sustainable hydrogen generation. The fundamental equation, 2 H2O → 4 H+ + O2 + 4 e−, describes the light-dependent reactions occurring in all photosynthetic organisms. While most organisms stop at oxygen evolution, a few, notably the green alga *Chlamydomonas reinhardtii* and certain cyanobacteria, have evolved a second, specialized step in their dark reactions. Here, dedicated hydrogenases located within the chloroplasts efficiently reduce the protons and electrons produced from water splitting to form H2 gas, directly converting solar energy into hydrogen fuel.
Recognizing this profound potential, significant efforts have been undertaken to genetically modify cyanobacterial hydrogenases to enhance their efficiency in generating H2 gas, even in the presence of oxygen, which traditionally inhibits these enzymes. Similar groundbreaking efforts are also being pursued with genetically modified algae grown in carefully controlled bioreactors, aiming to create scalable, biological systems that can produce hydrogen sustainably. These innovative biohydrogen approaches could revolutionize renewable energy by leveraging and optimizing nature’s own elegant solutions.

14. **Thermochemical Water Splitting: Heat-Driven Hydrogen for a Sustainable Future**Beyond biological pathways, another intriguing avenue for sustainable hydrogen production involves thermochemical water splitting. This process, as its name suggests, harnesses heat to decompose water into its fundamental components, hydrogen and oxygen, without the need for electricity as in electrolysis. The simplified reaction, 2 H2O → 2 H2 + O2, represents a direct and efficient way to tap into high-temperature heat sources for hydrogen generation, potentially offering a very clean production method.
The scientific community has identified and evaluated over 200 distinct thermochemical cycles for water splitting, each involving a series of chemical reactions that regenerate the original reactants, creating a continuous loop. Prominent examples of these cycles include the iron oxide cycle, the cerium(IV) oxide–cerium(III) oxide cycle, the zinc zinc-oxide cycle, the sulfur-iodine cycle, the copper-chlorine cycle, and the hybrid sulfur cycle. Each of these cycles features unique operating conditions, materials, and efficiencies, requiring extensive research to optimize their performance and scalability.
These diverse thermochemical cycles have been meticulously evaluated for their commercial potential, specifically aiming to produce hydrogen and oxygen directly from water and heat without the explicit use of electricity. The primary advantage lies in their ability to utilize waste heat from industrial processes, concentrated solar power, or nuclear heat, thereby offering a potentially more energy-efficient and cost-effective route compared to electrical-intensive methods, especially in regions with abundant high-temperature heat sources.
Across the globe, numerous laboratories in countries such as France, Germany, Greece, Japan, and the United States are actively engaged in developing and refining thermochemical methods to produce hydrogen, with a particular focus on leveraging solar energy as the heat source. This convergence of water splitting with renewable solar thermal energy points towards a truly sustainable and environmentally friendly pathway for hydrogen production, promising to significantly reduce our reliance on fossil fuels and accelerate the transition towards a clean energy economy. The ongoing research in this field holds the key to unlocking a future powered by abundant, clean hydrogen.
As we conclude our comprehensive exploration of hydrogen, from its cosmic origins and fundamental atomic secrets to its diverse forms and the innovative methods of its production, it becomes clear that this simple element is anything but ordinary. Hydrogen stands at the precipice of a global energy transformation, not merely as a constituent of water or a fuel for stars, but as a critical component in building a sustainable future. The ongoing advancements in harnessing hydrogen, whether through advanced industrial processes or nature-inspired biohydrogen, paint a vivid picture of a world where clean, abundant energy is not a distant dream, but an achievable reality, driven by the universe’s prime element. The journey of hydrogen, in essence, mirrors our own quest for ingenuity and sustainability.