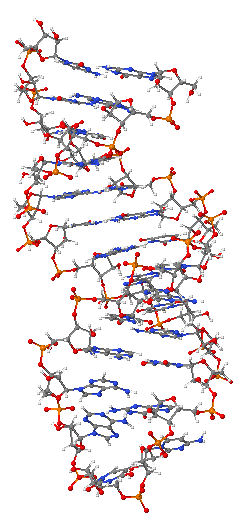
In the intricate tapestry of life, where genetic information dictates the very essence of existence, one molecule stands as a pivotal intermediary: messenger ribonucleic acid, or mRNA. This single-stranded molecule serves as the crucial bridge in the flow of genetic information, translating the static blueprint of DNA into the dynamic construction of proteins, the workhorses of every living cell. Understanding mRNA is not merely an academic exercise; it is fundamental to grasping the core processes that underpin all biological systems, from the simplest bacteria to the most complex multicellular organisms.
The journey of mRNA, from its conceptualization by pioneering scientists in the mid-20th century to its modern-day applications in groundbreaking medical advancements, is a testament to the relentless pursuit of scientific knowledge. It begins deep within the cell’s nucleus, where genetic code resides, and extends into the cytoplasm, where the machinery of protein synthesis operates. This complex and highly regulated “life cycle” ensures that the right proteins are made at the right time, in the right place, underpinning cellular function and organismal health.
As we delve into the world of mRNA, we uncover a series of fascinating molecular events—transcription, intricate processing steps, precise transport mechanisms, and finally, translation—each critical to its function. These processes, while meticulously studied, continue to reveal layers of complexity and regulatory nuances. This exploration will illuminate the foundational principles governing mRNA, showcasing its remarkable journey and immense significance in the grand scheme of molecular biology.
1. **Messenger Ribonucleic Acid (mRNA): The Blueprint of Life’s Proteins**: At its most fundamental level, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that directly corresponds to the genetic sequence of a gene. Its defining role is to be read by a ribosome during the complex process of synthesizing a protein. The concept of mRNA was a groundbreaking development in molecular biology, first conceived by Sydney Brenner and Francis Crick in 1960 during a conversation with François Jacob. Its existence was subsequently identified and described independently in 1961 by a team including Brenner, Jacob, and Matthew Meselson, and another led by James Watson. It was Jacob and Jacques Monod who famously coined the name “messenger RNA” while preparing their findings for publication, perfectly encapsulating its role.
This “messenger” function is central to the central dogma of molecular biology, which describes the flow of genetic information in a biological system. Genetic information, initially stored in the sequence of nucleotides within DNA, is copied into an mRNA molecule. This mRNA then carries that specific genetic message out of the nucleus and into the cytoplasm, where it encounters ribosomes. Here, the mRNA sequence acts as a precise template, guiding the assembly of amino acids into specific proteins, thereby translating the genetic code into functional cellular components.
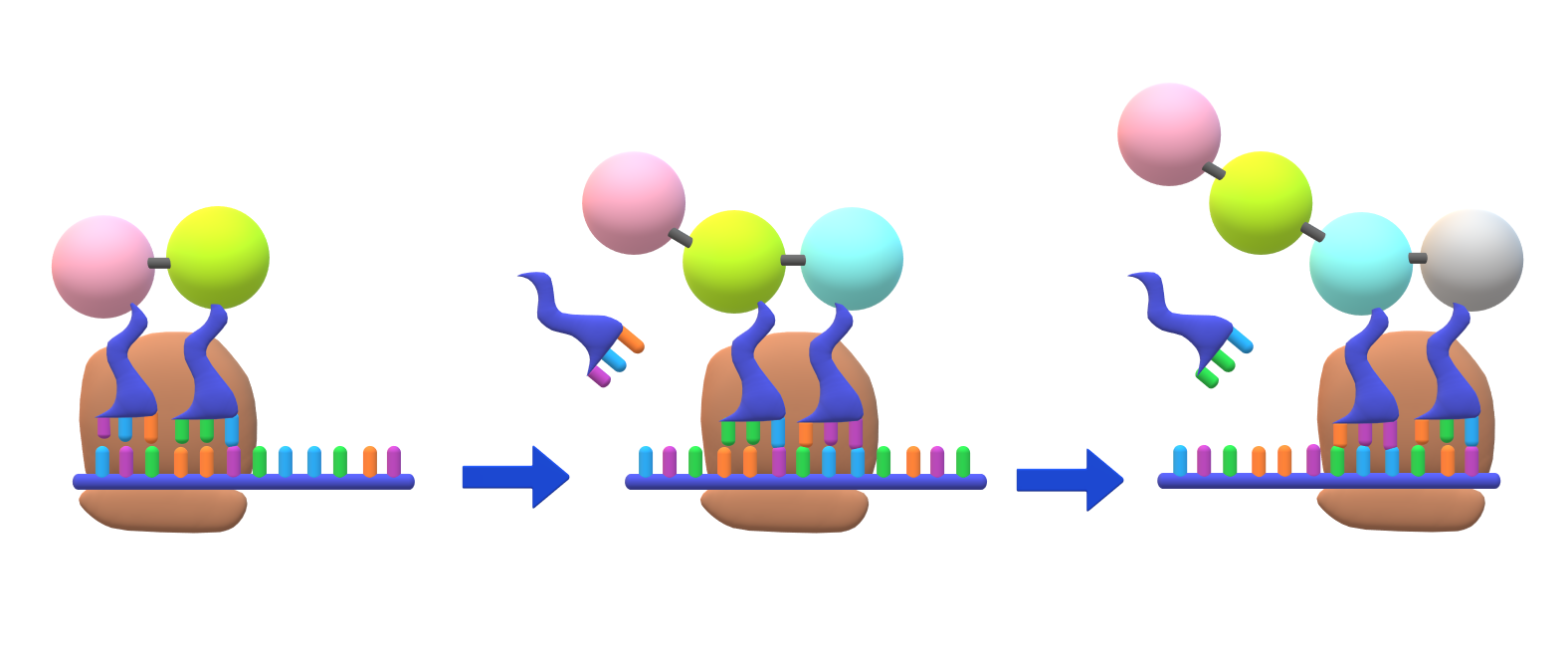
The sequence of nucleotides in mRNA is arranged into codons, each consisting of three ribonucleotides. With the exception of stop codons, which signal the termination of protein synthesis, each codon codes for a specific amino acid. The intricate translation process relies on two other critical types of RNA: transfer RNA (tRNA), which recognizes the codons and delivers the corresponding amino acid, and ribosomal RNA (rRNA), an integral component of the ribosome’s protein-manufacturing machinery. This cooperative interplay ensures the accurate and efficient synthesis of proteins, vital for all cellular activities.
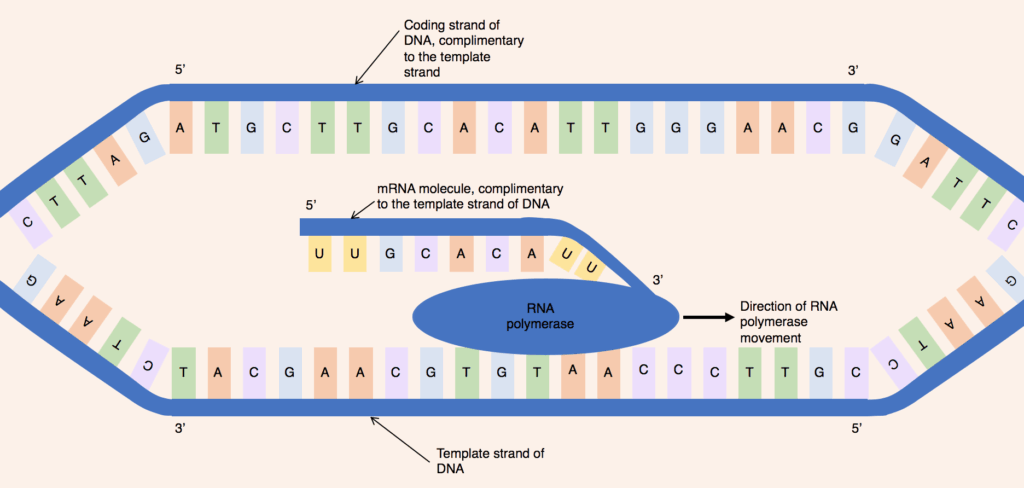
2. **Transcription: From DNA to mRNA**: The brief existence of an mRNA molecule commences with transcription, a fundamental process where RNA is meticulously copied from a DNA strand within the cell’s nucleus. During this crucial stage, an enzyme known as RNA polymerase diligently makes a copy of a specific gene from the DNA, producing the initial mRNA transcript as needed by the cell. This process, while broadly similar, exhibits slight variations between eukaryotic and prokaryotic organisms, reflecting differences in their cellular organization and regulatory complexities.
A notable distinction lies in the coupling of transcription and processing. In prokaryotic cells, RNA polymerase closely associates with DNA-processing enzymes during transcription, enabling processing to occur simultaneously with the synthesis of the RNA strand. However, the short-lived, unprocessed or partially processed product is initially termed precursor mRNA, or pre-mRNA. It is only upon its complete processing that it earns the designation of mature mRNA, ready for the subsequent stages of its life cycle, which ensures the integrity and functionality of the genetic message.
The template for mRNA synthesis is the complementary strand of tRNA, which astonishingly possesses a sequence identical to the anticodon sequence that the DNA binds to. This intricate dance of complementary base-pairing ensures that the genetic information is accurately transferred from the DNA template to the nascent mRNA molecule. This precise copying is paramount, as any errors introduced at this stage can have profound implications for the resulting protein, underscoring the high fidelity of cellular processes.
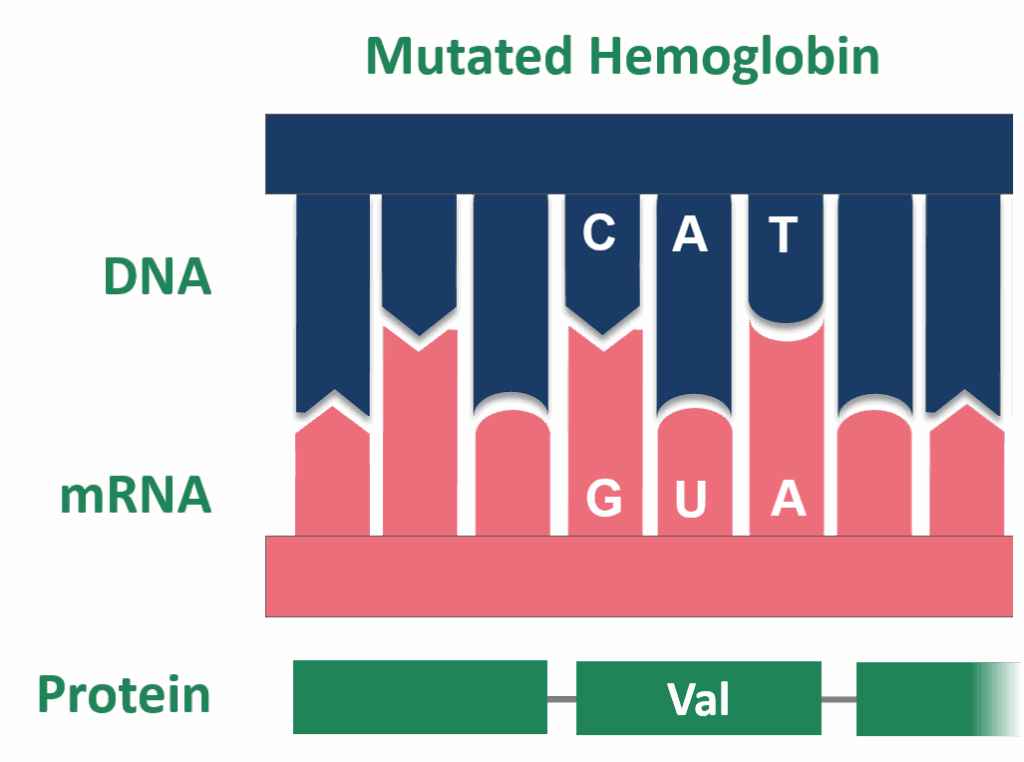
3. **The Uracil Substitution: A Tale of Evolutionary Advantage**: One of the most distinguishing features of mRNA, setting it apart from DNA, is the consistent use of uracil (U) in place of thymine (T). In the context of transcription, uracil serves as the complementary base to adenine (A), taking on the role that thymine plays in DNA. Consequently, when a template strand of DNA is utilized to construct an RNA molecule, every instance of thymine in the DNA sequence is faithfully replaced with uracil in the newly synthesized RNA strand. This substitution is not arbitrary; it allows the mRNA to accurately carry the appropriate genetic information from the DNA to the ribosome for the subsequent process of translation.
From a natural history perspective, compelling evidence suggests that uracil emerged first in evolutionary timelines, preceding thymine. The influential RNA World hypothesis posits that life on Earth initially began with RNA molecules, predating the evolution of DNA genomes and the emergence of coded proteins. This fascinating theory highlights RNA’s versatility, capable of both storing genetic information and catalyzing biochemical reactions, roles now primarily divided between DNA and proteins.
The evolutionary transition from uracil to thymine in DNA is believed to have conferred significant advantages. The presence of thymine, which includes a methyl group absent in uracil, is thought to have substantially increased DNA stability. This enhanced stability would have been crucial for maintaining genetic integrity over generations. Furthermore, this substitution is also considered to have improved the overall efficiency of DNA replication, facilitating the more robust and reliable propagation of genetic information in increasingly complex life forms, marking a pivotal moment in the biochemical evolution of genetic material.
4. **Eukaryotic Pre-mRNA Processing: Essential Refinement**: The journey of mRNA in eukaryotic cells is significantly more intricate than in their prokaryotic counterparts, necessitating several essential processing steps before it can be transported to the cytoplasm and subsequently translated by the ribosome. Unlike non-eukaryotic mRNA, which is often considered mature upon transcription with rare exceptions, eukaryotic pre-mRNA undergoes extensive post-transcriptional modifications. These rigorous processing steps are crucial for ensuring the stability, functionality, and accurate translation of the genetic message, adapting it for the more compartmentalized and complex environment of eukaryotic cells.
This elaborate processing serves multiple vital purposes, preparing the mRNA for its precise role outside the nucleus. It involves the removal of non-coding segments, the addition of protective chemical caps, and the attachment of a unique tail structure. These modifications collectively act as quality control mechanisms, verifying the integrity of the genetic message and safeguarding it from premature degradation. Without these critical steps, the nascent mRNA would be unable to leave the nucleus, effectively recognized by the ribosomal machinery, or would quickly be destroyed in the highly active environment of the cytoplasm, rendering the genetic information unusable.
The extensive processing of eukaryotic pre-mRNA represents a sophisticated regulatory layer in gene expression, allowing for greater complexity and control. This multi-step refinement ensures that only fully prepared and functional mRNA molecules proceed to direct protein synthesis. It also provides opportunities for cellular regulation, influencing when, where, and how much protein is produced from a given gene, highlighting the precision inherent in eukaryotic molecular biology.
5. **RNA Splicing: Excising the Non-Coding Segments**: A hallmark of eukaryotic gene expression is RNA splicing, an extensive and precise processing event that dramatically refines pre-mRNA into its mature form. This crucial mechanism involves the meticulous removal of specific regions within the pre-mRNA molecule known as introns, or sometimes referred to as outrons, which are non-coding sequences. Simultaneously, the remaining coding regions, known as exons, are accurately joined together. This seamless excision and ligation process is indispensable, ensuring that the final mRNA molecule contains only the sequences directly relevant for protein synthesis.
The significance of RNA splicing extends beyond simply trimming unwanted genetic material. It ensures the fidelity of the protein product by removing sequences that would otherwise disrupt the correct reading frame or introduce premature stop codons, leading to truncated or non-functional proteins. Moreover, alternative splicing, though not explicitly detailed here, is a broader concept where different combinations of exons can be joined, allowing a single gene to code for multiple, distinct protein variants. This mechanism dramatically expands the proteomic diversity of an organism from a relatively smaller number of genes, highlighting the efficiency and adaptability of eukaryotic genetic systems.
The precision required for splicing is immense, as a single nucleotide error can lead to a frameshift mutation, rendering the entire downstream protein sequence incorrect. This complex process is orchestrated by a sophisticated machinery involving various RNA and protein components. Its proper functioning is critical for cellular health, and dysregulation of splicing has been implicated in numerous human diseases, underscoring its profound biological and clinical importance.
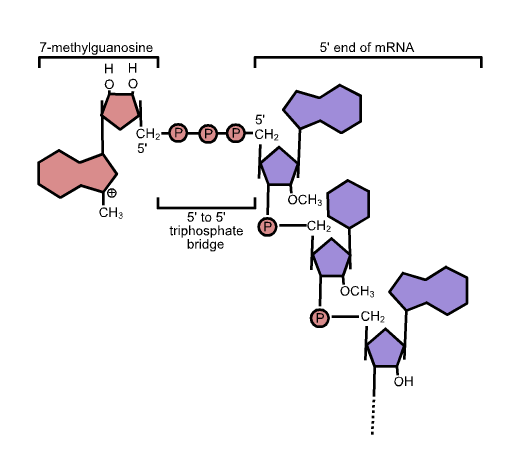
6. **The 5′ Cap: A Crucial Bookmark for Eukaryotic mRNA**: Shortly after the commencement of transcription, a unique and highly modified guanine nucleotide, known as the 5′ cap, is covalently added to the “front” or 5′ end of a eukaryotic messenger RNA molecule. This distinctive structure is also referred to by several other names, including an RNA cap, an RNA 7-methylguanosine cap, or an RNA m7G cap, all pointing to its specific chemical modification. The 5′ cap is formed by a terminal 7-methylguanosine residue, which is uniquely linked through a 5′-5′-triphosphate bond to the very first transcribed nucleotide of the mRNA strand. Its presence is not merely decorative; it is absolutely critical for multiple subsequent steps in the mRNA’s life cycle.
One of the primary and most vital functions of the 5′ cap is its role in the recognition of the mRNA by the ribosome. Without this distinctive cap, the ribosomal machinery responsible for protein synthesis would be unable to efficiently bind to the mRNA molecule, effectively halting translation. Furthermore, the 5′ cap provides crucial protection to the mRNA from degradation by exonucleases, enzymes that break down RNA molecules starting from their ends. This protective function significantly contributes to the stability and longevity of the mRNA, allowing it to remain intact long enough to direct the synthesis of multiple protein copies.
The addition of the 5′ cap is a fascinating example of tightly coupled molecular processes. It occurs co-transcriptionally, meaning it happens almost simultaneously with transcription, such that each process influences the other. An enzymatic complex, intricately associated with RNA polymerase, binds to the 5′ end of the nascent mRNA being synthesized. This complex then catalyzes the multi-step biochemical reactions required for mRNA capping, ensuring that the message is immediately marked and protected as it emerges from the DNA template. This synchronized activity underscores the elegant efficiency of cellular regulatory mechanisms.
7. **mRNA Editing: Modifying the Message**: In certain instances, an mRNA molecule undergoes a fascinating post-transcriptional modification known as editing, a process that alters its nucleotide composition. This remarkable mechanism allows for a direct change in the genetic information encoded within the mRNA, potentially leading to the production of different proteins from the same gene depending on the tissue or cellular context. Unlike typical gene expression, where DNA dictates RNA, and RNA dictates protein, editing introduces an additional layer of complexity and regulatory control, demonstrating the dynamic nature of genetic information flow.
A prominent example in humans is the apolipoprotein B mRNA. This specific mRNA is edited in some tissues, but notably, not in others. The consequence of this editing is the creation of an early stop codon within the mRNA sequence. Upon subsequent translation by the ribosome, this premature stop codon results in the production of a shorter protein compared to the full-length version produced from the unedited mRNA. This tissue-specific modification allows for the generation of distinct functional isoforms of apolipoprotein B from a single gene, each tailored to the needs of different biological environments.
Another extensively characterized form of mRNA editing is A-to-I (adenosine to inosine) editing, which is meticulously carried out by double-strand specific adenosine-to-inosine editing (ADAR) enzymes. This type of editing can occur within both the open reading frame, which directly codes for proteins, and the untranslated regions (UTRs) of the mRNA. By altering the nucleotide sequence, A-to-I editing can change the structural properties of the mRNA itself and, if in the coding region, lead to amino acid substitutions in the resulting protein. While essential for development, the precise and full range of roles played by this sophisticated editing mechanism are still subjects of active research, continually revealing new insights into its impact on cellular function and regulation.
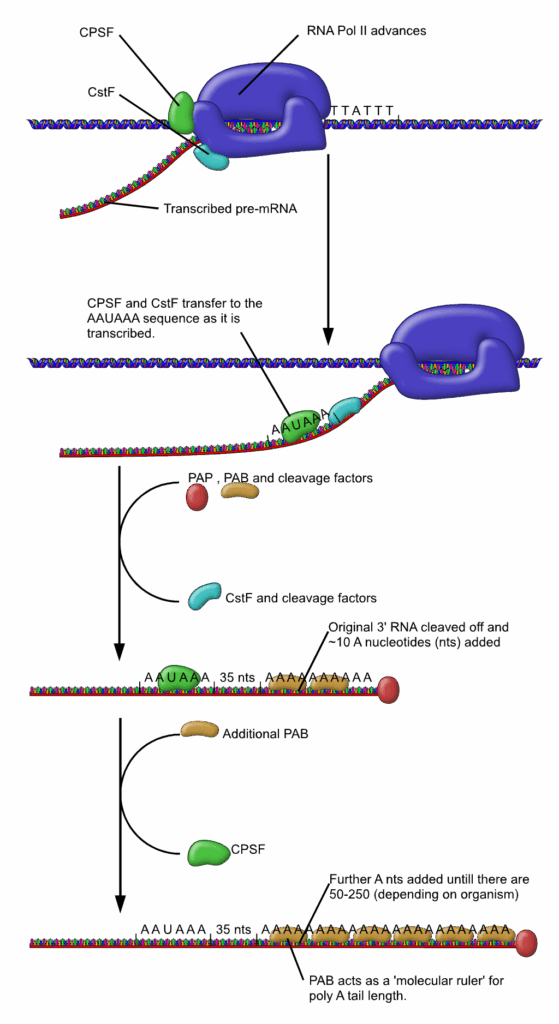
8. **Polyadenylation: The 3′ End’s Critical Tail**: As the journey of messenger RNA progresses in eukaryotic cells, an essential modification known as polyadenylation takes place. This process involves the covalent linkage of a polyadenylyl moiety to the molecule, typically at its 3′ end. This modification also commonly features short stretches of uridine, or oligouridylation, further highlighting the diversity of 3′ end modifications.
The poly(A) tail, with bound proteins, protects mRNA from exonuclease degradation. It is crucial for transcription termination, nuclear export, and efficient translation. While it impedes degradation in eukaryotes, poly(A) tails in prokaryotes surprisingly facilitate exonucleolytic breakdown.
Occurring during or immediately after transcription, an endonuclease complex cleaves the mRNA chain. Subsequently, polyadenylate polymerase adds approximately 250 adenosine residues to the new 3′ end. This process can yield multiple polyadenylation variants from a single mRNA, showcasing regulatory diversity.
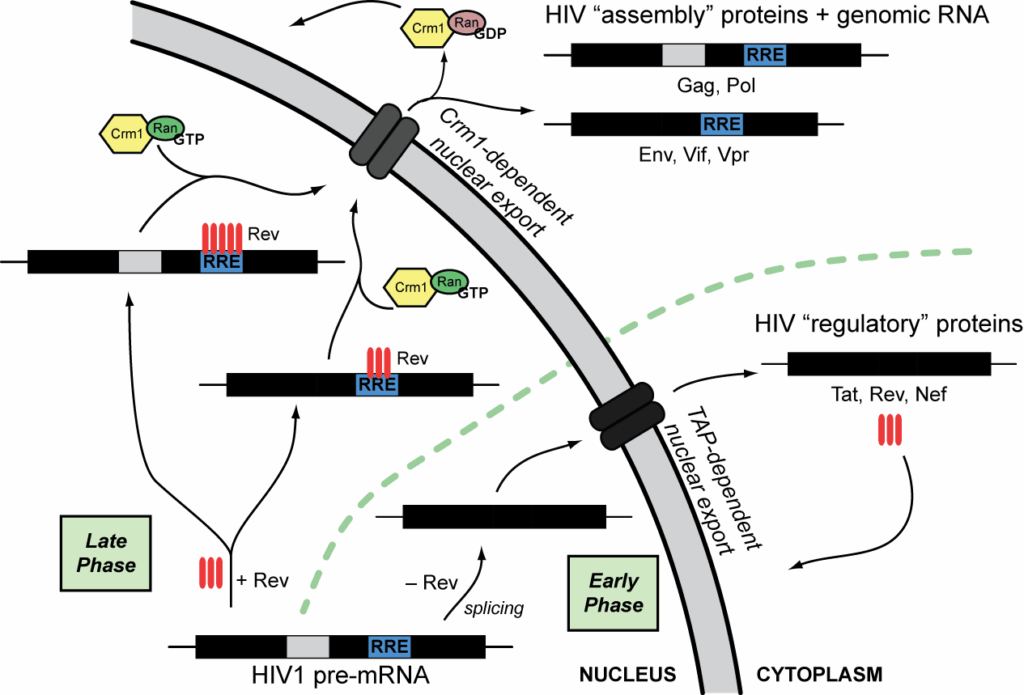
9. **mRNA Transport: Navigating the Cellular Labyrinth**: Eukaryotic mRNA requires precise transport from the nucleus to the cytoplasm, a necessity due to the compartmentalized nature of transcription and translation. This export is an actively regulated process, influenced by various cellular signaling pathways.
Mature mRNAs are identified by their processed modifications and exported through the nuclear pore. This journey is facilitated by binding to cap-binding proteins CBP20 and CBP80, and the transcription/export complex (TREX). Multiple export pathways underscore the complexity and regulatory layers involved.
In cells with intricate structures, such as neurons, certain mRNAs are transported to specific subcellular destinations like dendrites, where translation occurs at localized polyribosomes. Examples include Arc/Arg3.1 mRNA, which targets active synapses based on NMDA receptor signals, and β-actin mRNA, which moves in response to external stimuli. These mRNAs often contain “zip codes” for precise localization, ensuring proteins are synthesized where needed.
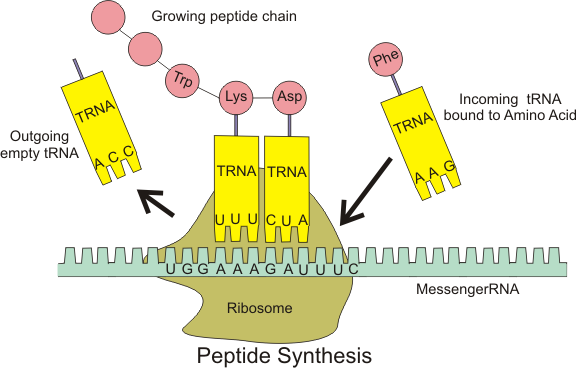
10. **Translation: The Protein Assembly Line**: The culmination of mRNA’s journey is translation, where its genetic information is decoded by ribosomes to synthesize specific proteins. The fundamental process differs notably between prokaryotic and eukaryotic systems.
In prokaryotes, transcription and translation are coupled, with ribosomes initiating protein synthesis almost immediately after transcription begins. This co-transcriptional coupling allows for a rapid and efficient cellular response to changing conditions.
Eukaryotic translation, conversely, is not directly coupled to transcription, occurring only after mRNA processing and nuclear-to-cytoplasmic transport. Ribosomes can be free-floating or directed to the endoplasmic reticulum for specific protein synthesis. This uncoupling provides additional regulatory layers, allowing for precise control over protein production independent of transcription rates.
The journey through mRNA’s advanced processing, intricate functions, and groundbreaking applications reveals a molecule of extraordinary versatility and immense therapeutic promise. From the precise tailoring of its message through polyadenylation and transport to its meticulously controlled degradation, mRNA embodies the cellular intelligence that ensures life’s complex machinery operates with precision. The recent triumphs in mRNA vaccine technology underscore not only decades of dedicated scientific inquiry but also the profound potential for this molecule to reshape future medical interventions, offering hope for new strategies against a myriad of human diseases.



