
Forty years ago, a quiet corner of atmospheric science suddenly grabbed the world’s attention. Scientists working diligently in the remote reaches of Antarctica stumbled upon something profoundly unsettling: a vast and unexpected thinning, a ‘hole,’ in the vital ozone layer high above the South Pole. This wasn’t just another scientific anomaly; it was a stark warning about humanity’s impact on a critical natural shield, sparking a global environmental emergency and, remarkably, a swift, coordinated response that stands as a beacon of international cooperation today.
The ozone layer, nestled in the stratosphere some 15–30 kilometers above our heads, plays an indispensable role in protecting life on Earth. It acts like a natural filter, absorbing a large amount of the sun’s harmful ultraviolet (UV) radiation before it reaches the surface. Without this protective shield, or with a significant gap in it, the increased UV exposure would have dire consequences for human health, leading to higher rates of skin cancer, cataracts, and immune system damage. It would also disrupt delicate ecosystems, from damaging plant tissues and reducing crop yields to impacting crucial life forms like plankton at the base of ocean food chains. The discovery of the ozone hole in 1985, therefore, wasn’t merely an academic finding; it was a matter of planetary survival.
Drawing on decades of dedicated research and insights from the very scientists who made this groundbreaking discovery, we can trace the fascinating and crucial story of the ozone hole. This journey takes us from the initial quiet data collection in Antarctica to the complex atmospheric chemistry unraveling far above the surface, the rapid political mobilization across the globe, and the ongoing effort to ensure the ozone layer fully recovers. It’s a powerful narrative that highlights the importance of robust science, clear communication, and the extraordinary potential of the world community to come together in the face of a shared threat.

1. **The Initial Shocking Discovery Over Antarctica**: On May 16, 1985, a paper published in the journal Nature revealed something unprecedented. A team at the British Antarctic Survey (BAS)—Joe Farman, Brian Gardiner, and Jonathan Shanklin—presented clear evidence that a hole had appeared in the ozone layer above Antarctica. For years, they had been quietly collecting and analyzing data using a Dobson ozone spectrophotometer, a relatively old device, simply trying to better understand the atmosphere over the continent.
Their data, however, began showing troubling, unexplained patterns starting in 1981. Jonathan Shanklin recalled feedback suggesting the data was “falling off the graph,” and it was “starting to look as if something was amiss.” Initially, they questioned if their instrument was malfunctioning, bringing a brand new Dobson to Antarctica the following summer. But the unexpected readings persisted, prompting Shanklin to compile data from the previous decade, initially collected by Joe Farman.
By going back through the historical records, Shanklin was able to confirm that the ozone depletion was not a one-off event but systematic. Each year in the springtime, the ozone levels were a little bit less than the previous year. This careful, long-term observation by the BAS team, working patiently with their “creaky-looking device,” led to one of the greatest geophysical discoveries of the 20th century, one that would fundamentally change how the world viewed environmental threats.
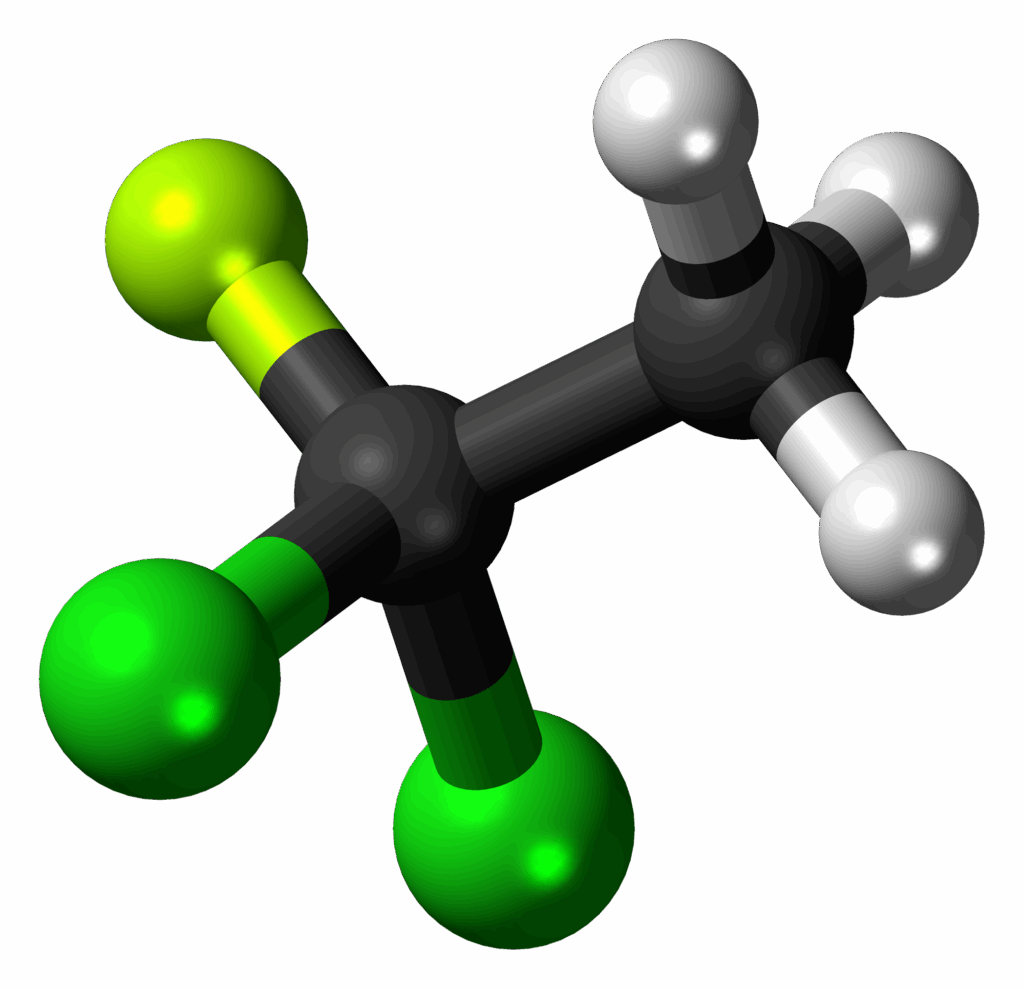
2. **Early Scientific Warnings About CFCs**: The discovery of the ozone hole didn’t happen in a vacuum; it was preceded by growing scientific concern for more than a decade regarding the potential harm caused by a class of fluorinated chemicals known as chlorofluorocarbons, or CFCs. As far back as 1974, chemists Mario Molina and Sherwood Rowland had already highlighted the perils of CFCs for stratospheric ozone.
Their foundational work built upon earlier studies, such as those by James Lovelock. Lovelock had invented a device called an electron capture detector, which made it possible for the first time to detect extremely low concentrations of atmospheric chemicals. This technological leap allowed scientists like Molina and Rowland to track the presence of CFCs in the atmosphere and investigate their fate.
Molina and Rowland’s crucial contribution was showing *how* CFCs, chemicals valued by industry for their stability and inertness, could break down high in Earth’s atmosphere. They revealed that when CFCs reached the stratosphere, they released chlorine atoms. These chlorine atoms were not inert; they were highly reactive and could participate in reactions that catalytically destroyed ozone molecules, thus posing a threat to the vital protective layer.

3. **The Widespread Adoption and Versatile Uses of CFCs**: To understand the scale of the problem, it’s important to realize just how pervasive CFCs had become by the late 20th century. Their story begins earlier, in 1930, when Thomas Midgley Jr., a mechanical engineer at General Motors, publicly demonstrated the safety of a new gaseous compound he had developed. Intended as a safer alternative to toxic refrigerant gases like ammonia, this emerging chemical was non-flammable, non-toxic, and odorless, qualities that made it incredibly appealing for commercial use.
That same year, General Motors and DuPont collaborated to form the Kinetic Chemical Company, which would produce this revolutionary substance under the trade name Freon, DuPont’s brand for CFCs. The adoption was rapid; by 1935, millions of refrigerators using Freon-12 had been sold by Frigidaire, General Motors, and DuPont. The perceived safety of CFCs was so compelling that city governments across the United States began naming Freon as the only coolant permitted for use in public buildings, solidifying its place in essential infrastructure.
By the 1950s and 1960s, the versatility of CFCs led to their incorporation into an even wider array of products. Beyond refrigeration and air conditioning, their non-flammable, non-explosive, and non-toxic properties made them ideal as propellants in aerosol sprays, blowing agents for Styrofoam, solvents for cleaning, and even in fire extinguishers. This broad application meant that CFCs were deeply embedded in industrial processes and consumer goods, becoming incredibly profitable, reportedly raking in about a billion dollars a year in the U.S. at their peak use.
4. **The Fundamental Science: How CFCs Destroy Ozone**: The core scientific discovery that linked CFCs to ozone depletion was understanding the chemical reactions that occur high in the stratosphere. While seemingly inert and harmless at ground level, when CFC molecules rise into the upper atmosphere, they encounter intense ultraviolet (UV) radiation from the Sun.
This powerful UV radiation has enough energy to break the chemical bonds within the stable CFC molecules, specifically releasing highly reactive chlorine atoms. Once liberated, a single chlorine atom doesn’t just react with one ozone molecule (O₃) and stop; instead, it initiates a catalytic cycle. The chlorine atom reacts with an ozone molecule, destroying it and forming chlorine monoxide (ClO) and an oxygen molecule (O₂). The chlorine monoxide molecule is then free to react with another ozone molecule or an oxygen atom, regenerating the chlorine atom and allowing the cycle to repeat.
This catalytic process means that a single chlorine atom released from a CFC molecule can participate in tens of thousands of ozone destruction reactions before it is eventually removed from the stratosphere. This efficient, repeating destruction mechanism is why even seemingly small concentrations of CFCs released at the surface could, over time, cause significant thinning of the ozone layer globally, particularly in the upper stratosphere where UV radiation is strongest.

5. **Why the Hole Formed Specifically Over Antarctica**: While CFCs were destroying ozone globally, the dramatic “hole” was observed specifically over Antarctica. Scientists quickly sought to understand why this region was uniquely vulnerable. It turned out that extremely cold atmospheric conditions and the formation of polar stratospheric clouds play a crucial, accelerating role in ozone depletion in the Antarctic spring.
During the long, dark Antarctic winter, temperatures in the stratosphere can drop below -108° F (-78° C). These frigid temperatures allow polar stratospheric clouds, sometimes called nacreous clouds, to form. These clouds are not typical water clouds; they are composed of ice crystals and other frozen particles that provide surfaces upon which key chemical reactions can occur. Critically, these ice crystal surfaces allow otherwise relatively inactive chlorine compounds, derived from the breakdown of CFCs, to be converted into highly reactive forms of chlorine and bromine.
Furthermore, these cold conditions lead to the removal of nitrogen compounds, which would normally help to regulate or deactivate the ozone-destroying chlorine. When spring arrives and sunlight returns to the polar region after the long winter, this stored, highly reactive chlorine is suddenly exposed to UV radiation. This triggers a rapid, runaway chain reaction where the reactive chlorine molecules rapidly break down ozone, leading to the sudden and dramatic thinning observed as the ozone hole during the Antarctic spring (typically August to October).

6. **The Vienna Convention: Laying the Groundwork for Action**: The growing scientific evidence regarding ozone depletion, culminating in the shocking discovery of the Antarctic ozone hole in 1985, galvanized international concern. This new understanding underlined the need for coordinated global action, as atmospheric chemicals know no national borders. The initial steps toward a global response actually began before the definitive paper on the ozone hole was published.
Recognizing the potential threat, governments had already begun discussing a collective plan to stop ozone depletion. In March 1985, just weeks prior to the publication of the paper by Farman’s team, more than 20 countries convened and signed the Vienna Convention for the Protection of the Ozone Layer. This was a crucial first step, representing a formal international recognition of the issue.
The Vienna Convention itself did not mandate specific, legally binding reductions in ozone-depleting substances. Instead, it served as a framework agreement. It urged governments to control emissions of ozone-depleting substances (ODS) “to the maximum extent practicable” and, importantly, committed nations to cooperation in research and monitoring. It laid the essential political and diplomatic groundwork for a future, more robust and legally binding agreement to be negotiated, setting the stage for the landmark treaty that would follow just two years later.

7. **The Montreal Protocol: A Landmark Global Agreement**: The definitive scientific proof of the ozone hole and the clear link to CFCs provided the undeniable impetus for stronger, legally binding international action. Building rapidly on the foundation laid by the Vienna Convention, the world community moved with unprecedented speed. In 1987, just two years after the ozone hole discovery was reported, a new, much stronger agreement was adopted: the Montreal Protocol on Substances that Deplete the Ozone Layer.
This treaty was revolutionary. It committed signatory countries to freeze the production and consumption of certain CFCs at 1986 levels and then systematically phase them out according to a specified schedule. It was a dynamic agreement designed to evolve as scientific understanding improved, crucially including precautions that would allow it to regulate ozone-depleting chemicals that might be developed or identified in the future.
The adoption of the Montreal Protocol marked a pivotal moment in environmental history. It demonstrated that nations, even in a divided world, could come together quickly and decisively to address a global environmental crisis based on scientific evidence. The fact that it was signed by 197 countries and the European Union, ultimately becoming the only UN treaty ratified by every single country on Earth, speaks volumes about the perceived urgency and the collective will to act on this specific threat. It was hailed by former UN Secretary-General Kofi Annan as “perhaps the single most successful international agreement to date.”
Building rapidly on the foundation laid by the Vienna Convention, the world community demonstrated remarkable agility. In 1987, just two years after the alarming discovery of the ozone hole was formally reported, a new, much more potent agreement was forged: the Montreal Protocol on Substances that Deplete the Ozone Layer. This was a pivotal moment, marking a shift from recognizing the problem to legally committing to solving it on a global scale.
This treaty was nothing short of revolutionary in its approach and ambition. It required signatory countries to freeze the production and consumption of certain CFCs at their 1986 levels, establishing a clear baseline for action. More importantly, it mandated a systematic phase-out of these harmful chemicals according to a defined schedule, setting concrete targets for reduction and eventual elimination. The protocol was also designed to be flexible and adaptable, crucially including provisions that would allow it to regulate other ozone-depleting chemicals that might be identified or developed in the future, ensuring its relevance over time as scientific understanding evolved.

8. **The Implementation and Structure of the Montreal Protocol**: The Montreal Protocol didn’t just set targets; it outlined a strategic path for achieving them, a structure that scholars have studied extensively for its effectiveness. It was organized around a rare strategy in public policy: “start gently, learn by doing, win the trust of stakeholders, then scale up.” This pragmatic approach allowed nations and industries to adapt gradually while building momentum towards stricter controls as alternatives became available and the science solidified.
A key component of the protocol’s structure was its reliance on regular science and technology assessments. These assessments, conducted by leading experts, were central to informing discussions about how best to phase down and eventually phase out ozone-depleting substances (ODS). By grounding policy decisions in the latest scientific understanding and technological feasibility, the protocol maintained credibility and ensured that its requirements were both ambitious and achievable, fostering cooperation rather than resistance.

9. **Financial Assistance and Compliance under the Protocol**: A critical element distinguishing the Montreal Protocol was its tiered approach to implementation, acknowledging the varying capacities of different nations. High-income countries were expected to lead the way in taking swift and meaningful action, demonstrating commitment and helping to smooth the transition for industries. Crucially, this leadership included actively getting industry on board, recognizing their essential role in developing and adopting alternative substances.
Recognizing that developing nations would face greater challenges in transitioning away from ODS, the protocol established a mechanism for financial assistance. High-income countries provided funding to low- and middle-income countries (LMICs), enabling them to also phase out the harmful chemicals without hindering their economic development. This commitment to shared responsibility and assistance was vital in achieving the treaty’s universal ratification and ensuring equitable participation.
The protocol also featured a unique compliance system. Governments actively monitor each other’s performance and pay close attention to any nation not meeting its obligations. This peer-monitoring and accountability mechanism fostered transparency and encouraged compliance, further strengthening the treaty’s effectiveness and building a functional global partnership anchored in research, trust, and collective experience.

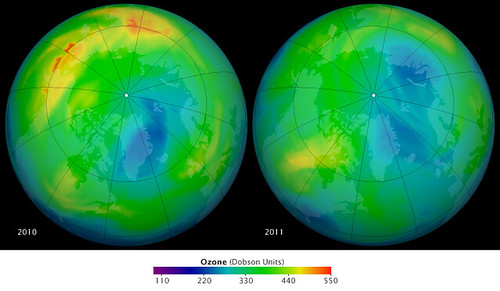
10. **Evidence of the Ozone Layer’s Recovery**: The collective action spurred by the Montreal Protocol has yielded remarkable results, demonstrating the power of international cooperation driven by science. As of 2024, the atmospheric levels of the main ozone-depleting substances have dramatically decreased; worldwide emissions are reported to be 99% lower than they were at their peak in 1989. This significant reduction is a direct consequence of the global phase-out efforts mandated by the treaty.
Scientists monitoring the atmosphere have observed clear signs of the ozone layer’s recovery. The Antarctic ozone hole, which once grew alarmingly large each spring, has shown a steady trend of shrinking in size over the last 40 years. This measured improvement is a testament to the effectiveness of phasing out CFCs and other harmful substances, validating the scientific models and policy interventions put in place decades ago.
Based on current projections and the observed decline in ODS concentrations, the ozone layer is now on track for substantial recovery. Scientists anticipate that the ozone layer will recover to pre-1980 levels by mid-century. This long-term outlook offers a hopeful perspective, showing that even major environmental damage caused by human activity can, over time and with sustained effort, be undone.
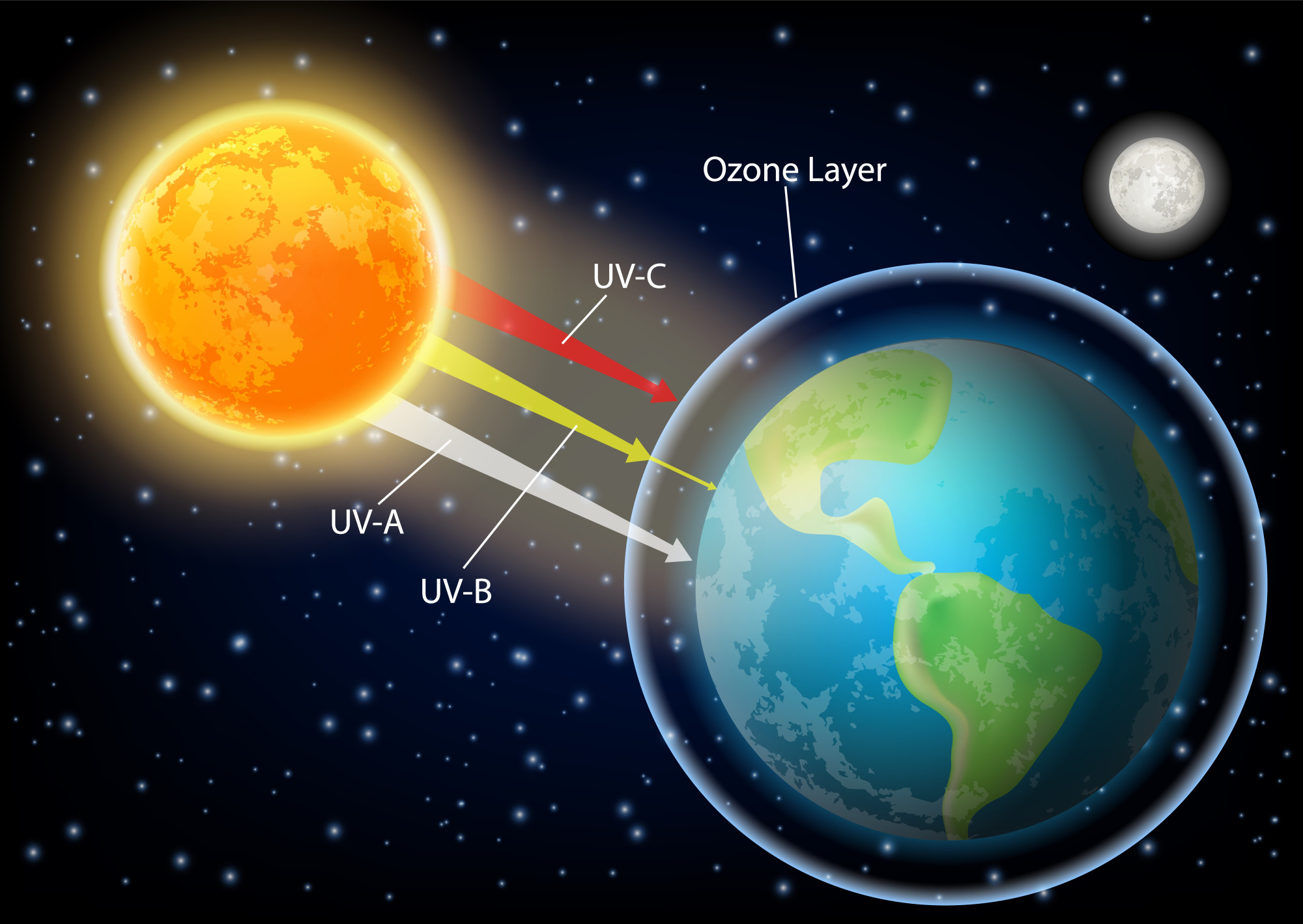
11. **Factors Influencing the Pace of Recovery**: While the recovery is underway and considered a success story, it is also a slow process. The rate of healing is influenced by several factors, including the long atmospheric lifetimes of many ozone-depleting chemicals. Substances like CFCs can persist in the stratosphere for more than 50 years, meaning their ozone-destroying potential lingers for decades even after production and emissions have ceased.
Furthermore, there is evidence suggesting that the recovery rate might be a bit slower than initially expected. Scientists are exploring the potential influence of interactions with climate change. The complex and changing climate system can affect atmospheric temperatures, circulation patterns, and other conditions in the stratosphere, which in turn may subtly alter how ozone forms and depletes, potentially impacting the pace at which the layer heals.
The inherent variability in the atmosphere from year to year also makes detecting the recovery challenging. Seasonal fluctuations and other natural phenomena can mask the slow, steady improvement driven by the reduction in ODS. This complexity requires continuous, careful monitoring and sophisticated analysis to accurately track the healing process and understand the full range of factors at play.
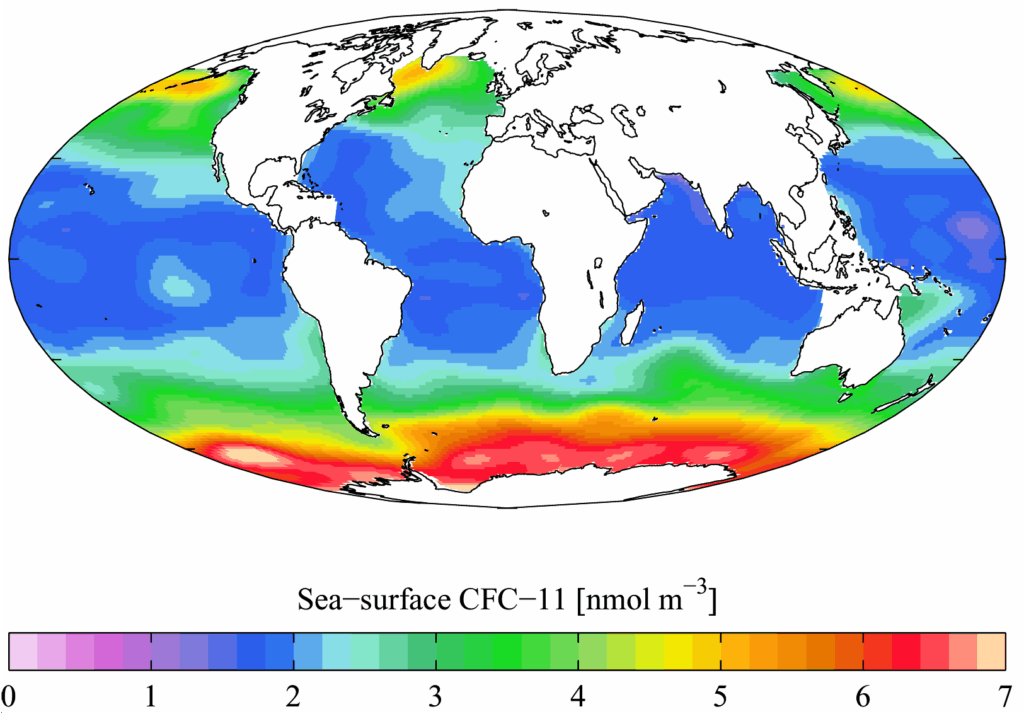
12. **Lingering Threats and Challenges to Full Recovery**: Despite the overall success of the Montreal Protocol, vigilance remains necessary to ensure the ozone layer’s full recovery. One ongoing challenge is the potential for rogue emissions of banned substances. In recent years, for example, unexpected emissions of CFC-11 were detected and traced, highlighting that compliance isn’t always perfect globally and requiring continued monitoring and enforcement.
Climate change itself presents a complex interaction with ozone recovery. Shifting atmospheric dynamics caused by rising greenhouse gas levels could influence stratospheric temperatures and circulation, potentially altering the patterns and speed of ozone recovery. Research into this feedbacks is ongoing to fully understand how global warming might impact the healing process.
Emerging technologies and activities also pose potential future threats that require careful assessment. Early research suggests that the increasing frequency of satellite and rocket launches could negatively impact the ozone layer, both during launch phases and upon satellite reentry. These developments underscore the need for continued scientific monitoring and potentially the need for the Montreal Protocol to adapt and regulate new substances or activities.

13. **The Montreal Protocol as a Model for Global Action**: The international response to the ozone crisis stands as a powerful testament to what can be achieved when science, diplomacy, and policy align effectively. The Montreal Protocol has not only put the ozone layer on the path to recovery but has also been estimated to have prevented millions of cases of skin cancer and other health issues by reducing harmful UV radiation exposure.
Its success is often contrasted with the slower progress in tackling other global environmental challenges, such as climate change, biodiversity loss, and pollution. Experts point to key elements that made the Montreal Protocol work: robust scientific evidence clearly identifying the cause and solution, clear communication of the threat, strong international cooperation leading to a universally ratified treaty, a flexible structure, financial mechanisms to support developing nations, and a commitment to ongoing assessment and compliance.
The phased approach—starting with achievable goals and gradually increasing ambition based on scientific advancements and technological feasibility—was particularly effective. The protocol demonstrated that even in a divided world, nations can come together quickly and decisively to address a shared environmental crisis when the threat is clear and a feasible path to action exists, offering valuable lessons for future global challenges.

14. **Comparing Ozone Action to Climate Change Efforts**: While the Montreal Protocol offers crucial lessons, the challenge of climate change is proving significantly more complex. As atmospheric chemists point out, the “climate story is much, much more difficult than the ozone story.” One key difference lies in the nature of the substances involved: ODS were produced at a limited number of facilities, had a narrow range of applications, and alternatives could be developed by the chemical industry.
Fossil fuels, by contrast, are high-volume, low-cost products deeply embedded in virtually every sector of the global economy since the Industrial Revolution. The scale of the required transition is vastly different, not just industrially but financially; the fossil fuel infrastructure is estimated to be worth some $40 trillion, compared to the roughly $1 billion value of the CFC production facilities in the 1980s.
Furthermore, the political and economic landscape for climate action has been more challenging. While emissions cuts did start gently, high-income countries have been slow to take the lead commensurate with their historical responsibility. The financial support pledged to LMICs for clean development has been insufficient. The fossil fuel industry, unlike the chemical industry in the 1980s which could profit from alternatives, has often actively resisted climate science and action, contributing to a lack of trust between parties. Despite these hurdles, agreements like the Paris Agreement are beginning to operate with increasing ambition over time, climate finance is slowly rising, and renewable energy is growing rapidly, suggesting that progress, however difficult, is being made.
The story of the ozone hole, from shocking discovery to global recovery efforts, is a powerful narrative of science informing action and nations uniting to solve a planetary-scale problem. While the path to healing is long and requires continued monitoring for new threats and interactions with climate change, the Montreal Protocol remains a shining example of what is possible when the world commits to robust science, clear communication, and mutual trust in the face of environmental crisis. It provides hope and crucial lessons as we navigate the even greater complexities of climate change and other pressing global environmental challenges. Though the economic models that underpin global resource use still need reform, the ozone success story demonstrates that addressing planetary threats is achievable.



